Abstract
Objective. Transcutaneous spinal cord stimulation (tSCS) has been gaining momentum as a non-invasive rehabilitation approach to restore movement to paralyzed muscles after spinal cord injury (SCI). However, its low selectivity limits the types of movements that can be enabled and, thus, its potential applications in rehabilitation. Approach. In this cross-over study design, we investigated whether muscle recruitment selectivity of individual muscles could be enhanced by multielectrode configurations of tSCS in 16 neurologically intact individuals. We hypothesized that due to the segmental innervation of lower limb muscles, we could identify muscle-specific optimal stimulation locations that would enable improved recruitment selectivity over conventional tSCS. We elicited leg muscle responses by delivering biphasic pulses of electrical stimulation to the lumbosacral enlargement using conventional and multielectrode tSCS. Results. Analysis of recruitment curve responses confirmed that multielectrode configurations could improve the rostrocaudal and lateral selectivity of tSCS. To investigate whether motor responses elicited by spatially selective tSCS were mediated by posterior root-muscle reflexes, each stimulation event was a paired pulse with a conditioning-test interval of 33.3 ms. Muscle responses to the second stimulation pulse were significantly suppressed, a characteristic of post-activation depression suggesting that spatially selective tSCS recruits proprioceptive fibers that reflexively activate muscle-specific motor neurons in the spinal cord. Moreover, the combination of leg muscle recruitment probability and segmental innervation maps revealed a stereotypical spinal activation map in congruence with each electrode's position. Significance. Improvements in muscle recruitment selectivity could be essential for the effective translation into stimulation protocols that selectively enhance single-joint movements in neurorehabilitation.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Spinal cord injury (SCI) is a life-altering event that leads to long-lasting motor impairment, and currently, there is no cure for paralysis (Armour et al 2016, National Spinal Cord Injury Statistical Center 2021). Spinal cord stimulation (SCS) has been gaining momentum as a neuromodulation intervention to restore movement to paralyzed areas below the injury (Minassian et al 2007b, Harkema et al 2011, Angeli et al 2018, Gill et al 2018, Wagner et al 2018, Rowald et al 2022). By selectively activating individual muscle groups at the appropriate phases of movement, epidural SCS (eSCS) can re-enable the performance of dexterous activities such as walking, cycling, swimming, and kayak paddling (Wagner et al 2018, Rowald et al 2022). However, the invasive nature of eSCS and the large group of experts needed with high associated costs may prevent eSCS from becoming an accessible therapy for the millions of people living with paralysis.
Transcutaneous SCS (tSCS) offers a promising non-invasive alternative to engage paralyzed muscles below the injury by activating the same neural structures via similar mechanisms as eSCS (Minassian et al 2007a, Ladenbauer et al 2010, Danner et al 2011). In conventional applications of tSCS, a single electrode is positioned over the T11/T12 interspinous ligament and centered at the midline (Minassian et al 2007a, Ladenbauer et al 2010, Danner et al 2011, Hofstoetter et al 2021a). In clinical applications of tSCS, continuous stimulation protocols at 30 Hz or 50 Hz are used to facilitate motor function and spasticity, respectively (Hofstoetter et al 2013, Gerasimenko et al 2015a, Hofstoetter et al 2020, 2021a). And recent work suggests that positive rehabilitative outcomes may also be achievable, albeit to a lesser degree than eSCS, through this non-invasive approach (Al'joboori et al 2020, Shapkova et al 2020, Inanici et al 2021, Samejima et al 2022). However, the low selectivity of tSCS compared to eSCS (Hofstoetter et al 2018) may limit the types and number of movements that can be enabled by tSCS and, thus, its potential applications in exercise-based rehabilitation strategies.
New findings revealed that positioning the cathode electrode at different locations can enable the preferential recruitment of either muscles ipsilateral to the stimulation site (Calvert et al 2019) or rostral vs caudal muscle groups (Krenn et al 2015, Sayenko et al 2015) in the lumbosacral region. However, repositioning an electrode to target different muscle groups within a rehabilitation session would significantly limit the feasibility of performing this approach in a clinical setting.
Emerging studies have indicated that multielectrode arrays positioned over the cervical spinal cord have the potential to preferentially target specific motor neuron pools (de Freitas et al 2021, 2022, Oh et al 2022). These studies suggest that it may also be possible to attain preferential activation of specific motor neuron pools in the lumbar spinal cord using one single multielectrode configuration. However, unlike the cervical spinal cord, where the posterior roots for flexor and extensor muscles are generally separated (Schirmer et al 2011, Greiner et al 2021), there is a significant overlap in the lumbar spinal cord (Sharrard 1964, Schirmer et al 2011, Hofstoetter et al 2021b, Rowald et al 2022). Therefore, a comprehensive investigation of the improvements in selectivity enabled by multielectrode arrays in the lumbar spinal cord is necessary. Additionally, while preferential activation of specific muscle groups has been reported, there is a lack of direct comparisons to quantify the magnitude of these improvements in recruitment selectivity compared to conventional tSCS approaches or different electrode configurations.
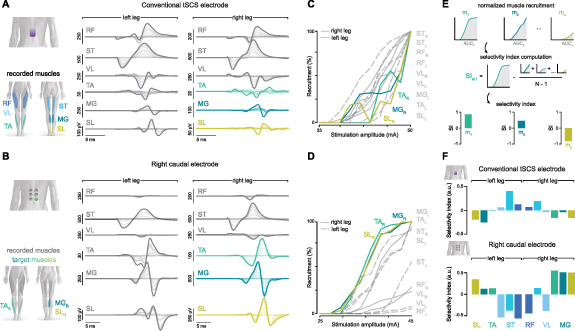
In this work, we demonstrate that it is possible to have a single experimental setup in a multielectrode configuration that enables the combination of independent observations of improvements in selectivity of proximal vs. distal as well as unilateral muscles. We aimed to understand whether we could exploit the organization of motor neuron pools in the spinal cord to identify stimulation sites that result in optimal recruitment selectivity for different key leg muscles. We studied evoked compound muscle action potentials of lower-limb muscles in response to stimulation over the T10-L1 vertebral segments using conventional and multielectrode configurations of tSCS (figures 1(A) and (B)). First, we analyzed recruitment curves for individual leg muscles (figure 1(C)) when the cathode was a single large electrode as in conventional tSCS (centered at T11/T12) or in one of six locations in a multielectrode configuration. We compared the recruitment selectivity enabled for each muscle at each cathode location and identified optimal electrode configurations that enabled the highest selectivity for that muscle.
Figure 1. Experimental framework to compare muscle recruitment selectivity by conventional and multielectrode tSCS. (A) The rostrocaudal and unilateral organization of leg muscles' motor neuron pools in the spinal cord may enable the selective recruitment of individual muscle groups by small diameter electrodes centered at the T11/T12 vertebrae. Modified from (Sharrard 1964, Wagner et al 2018). (B) We recorded leg muscle responses while pulses of tSCS were delivered at increasing stimulation amplitudes. (C) To quantify each muscle's recruitment curve, we computed peak-to-peak response amplitude across a range of increasing stimulation amplitudes. Scale bars: 100 μV and 10 ms. (D) We hypothesize that compared to the non-specific recruitment by conventional single electrodes, a small diameter electrode positioned rostrally and over the right side would enable the selective targeting of right leg proximal muscles (hip, thigh), while a right caudal electrode would target right distal muscles (ankle). RF: rectus femoris; VL: vastus lateralis; ST: semitendinosus; TA: tibialis anterior; MG: medial gastrocnemius; SL: soleus; R: right leg. Modified from (Bryson et al 2023).
Download figure:
Standard image High-resolution imageBased on the rostrocaudal anatomical distribution of the motor neuron pools that innervate leg muscles (Sharrard 1964, Wagner et al 2018, Hofstoetter et al 2021b) (figure 1(A)), we hypothesized that while conventional tSCS results in the non-selective recruitment of proximal and distal leg muscles, the multielectrode configuration would enable preferential mediolateral and rostrocaudal recruitment of leg muscles. For example, a right-side cathode centered rostrocaudally at the T10/T11 interspinous ligament (overlapping the L1-L3 spinal segments) would primarily recruit proximal muscles in the right leg, whereas a right-side cathode centered at the T12/L1 interspinous ligament (overlapping the L4-S3 spinal segments) would primarily recruit the right distal muscles (figure 1(D)).
Innovations in electrode positioning (Krenn et al 2015, Sayenko et al 2015, Calvert et al 2019, de Freitas et al 2021, 2022) and multi-site stimulation (Gerasimenko et al 2015b, Parhizi et al 2021, Samejima et al 2022) have recently gained interest in the field of tSCS. However, various studies have demonstrated that changes in body position, as well as the placement of anode and cathode electrodes (Roy et al 2012, Danner et al 2016, Masugi et al 2017, Salchow-Hömmen et al 2021), can significantly increase the likelihood to recruit motor efferent fibers directly. Presynaptic recruitment at the posterior root afferents is preferred for neurorehabilitation, as this mechanism allows for spared spinal circuits and supraspinal inputs to interact with the stimulation (Minassian et al 2016, Guiho et al 2021). Therefore, to facilitate the translation of our approach into a clinical setting and minimize the possibility of habituation effects resulting from continuous stimulation of efferent fibers (Dimitrijević et al 1972), it is crucial to conduct a comprehensive investigation into the activation mechanisms that underlie the observed improvements in muscle recruitment selectivity. We hypothesized that the improved selectivity achieved through the multielectrode configuration would be mediated by the activation of muscle recruitment via sensory afferents, as evidenced by post-activation depression of the evoked response.
Finally, we combined the recruitment probability of a given muscle at a specific cathode site with previously reported segmental innervation probabilities at different spinal segments to validate our results and provide a neuroanatomical map of recruited spinal segments by each electrode configuration. Gaining a better understanding of the neural mechanisms behind these improvements in muscle recruitment selectivity by spatially selective tSCS may expedite the development of non-invasive technologies that can re-enable a broad repertoire of dexterous movements in rehabilitation and daily life.
2. Methods
2.1. Participants and experimental setup
We recruited 19 neurologically intact participants (9 female, 10 male, average age 23.12 ± 4.34 years old, demographics in supplementary table 1) who gave their consent to take part in this study that was reviewed and approved by Washington University in St. Louis' Institutional Review Board. Two participants (1 male and 1 female) withdrew from the study due to discomfort with the stimulation. One participant recruited early in the study was excluded from the analysis due to the use of monophasic, rather than biphasic stimulation, for a total of 16 participants included in the analysis for all figure.
2.2. Study protocol
2.2.1. Vertebral segment identification and electrode placement
We identified target vertebral segments T11/T12 via manual palpation, with validation by a second experienced research team member. The posterior iliac crest was first identified, and a transverse line was traced to the midline. The spinous process intersecting with this line was labeled with a surgical skin marker as the L4 spinous process. Interspinous ligaments were identified by palpation and labeled from L3/L4 to T9/T10. The skin around the midline from T10 to L2 was prepared with abrasive gel (NuPrep®, Weaver and Co. USA) using a Q-tip® in circular motions and wiped afterward with alcohol pads.
For the conventional tSCS condition, we positioned a single 5 × 9 cm rectangular electrode (all tSCS electrodes are PALS Neurostimulation Electrodes, Axelgaard Manufacturing Co., Ltd, USA) centered over the interspinous ligament of T11/T12 (figure 1(A), top). Two interconnected 7.5 × 10 cm rectangular electrodes were placed on the abdomen bilaterally from the navel to serve as the return electrodes. All tSCS electrodes were treated with conductive spray (Signa® Spray, Parker Laboratories, Inc., USA).
Although palpation provides a useful starting point, anthropologic, clinical, and imaging studies have shown that variations in the number of vertebrae occur in 2%–23% of the population (Bailey and Carter 1938, Hahn et al 1992, Akbar et al 2010), and surface tSCS electrodes often need to be repositioned to achieve activation of the targeted muscles (Krenn et al 2015). To ensure the accurate position of the conventional tSCS electrode over the lumbosacral enlargement, effective stimulation of segmental afferents was neurophysiologically confirmed by the elicitation of posterior-root muscle (PRM) reflexes bilaterally in L2 to S2 innervated myotomes, i.e. in quadriceps, hamstrings, calves, and tibialis anterior muscles (Minassian et al 2016, Hofstoetter et al 2019, 2021a). If either proximal or distal muscles could not be recruited at comfortable stimulation amplitudes, we adjusted the electrode placement by moving it one segment up or down as necessary. Repositioning was performed on five participants (supplementary table 1), and the horizontal midline of the adjusted conventional electrode placement was then used as the rostrocaudal center for the multielectrode configuration (middle electrodes).
In the multielectrode condition, we positioned six 3.2 cm diameter round electrodes 3 cm lateral to the midline and centered at the T11/T12 interspinous ligament (or center of conventional electrode if repositioned, figure 1(A), bottom). Throughout the manuscript, we refer to the top, middle, and bottom row electrodes as the rostral, middle, and caudal electrodes, respectively. The top and bottom row electrodes were positioned to target the adjacent rostral and caudal interspinous ligaments, respectively. If electrode positions would overlap by this approach (particularly in shorter participants), the electrodes were placed 1 cm longitudinally from the center row. Overall, there was an approximate distance of 1–2 cm between electrodes in the longitudinal direction. In the multielectrode condition, only the abdominal electrode ipsilateral to the cathode was used as the return electrode.
2.2.2. Data acquisition
Wireless electromyography (EMG) sensors (Trigno® Avanti, Delsys Inc., USA) were placed bilaterally according to SENIAM guidelines on the rectus femoris (RF), vastus lateralis (VL), semitendinosus (ST), tibialis anterior (TA), medial gastrocnemius (MG), and soleus (SL) muscles. The skin was prepared using the same procedure as with the spinal electrodes, and electrodes were repositioned if the baseline noise was larger than 10 μV. An additional wireless sensor (Trigno® Analog Input Adapter, Delsys Inc., USA) was connected via a BNC cable to the biphasic stimulator's sync output for offline stimulation pulse alignment. Stimulation pulse triggering and amplitude were controlled via a data acquisition board (NI USB 6001, National Instruments, USA). EMG data was amplified using a data acquisition system (Trigno® Avanti Research+, Delsys Inc., USA; gain: 300; bandwidth 20–450 Hz), recorded at a sampling frequency of 2000 Hz, and displayed in real-time using a custom-built software written by our group in Python (v3.10).
2.2.3. Transcutaneous spinal cord stimulation
Paired pulse tSCS was delivered using an isolated constant current stimulator (DS8R, Digitimer Ltd, UK) with a pair of charge-balanced, anodic leading, biphasic pulses of 1 ms per phase duration and an inter-stimulus interval of 33.3 ms using a train generator (DG2A, Digitimer Ltd.). We delivered pulses of increasing stimulation amplitude to detect the motor threshold and saturation amplitude. We defined the motor threshold as the amplitude at which we observed a ⩾ 20 μV peak-to-peak response amplitude within a latency of 10–30 ms in any muscle. The saturation amplitude was defined as the amplitude at which we no longer saw an increase in response amplitude of the first recruited muscles or the maximum amplitude tolerated by participants, whichever was lower.
2.2.4. Recruitment curves
We performed recruitment curve recordings by increasing stimulation intensity from 5 mA below the motor threshold to the saturation amplitude, with eight steps between amplitudes for a total of ten stimulation amplitudes. Four repetitions of double-pulse stimulation were performed at each amplitude. Threshold detection and recruitment curve recordings were repeated for each of the seven electrode configurations (one in conventional tSCS, six in multielectrode tSCS). The testing order was randomized for the multielectrode configuration.
2.3. Data processing and analysis
Offline data analysis was performed in custom-built software written in Python and MATLAB® (The MathWorks Ltd, USA). Evoked responses were averaged across the four repetitions, with one average response waveform for each of the ten stimulation amplitudes. Evoked responses for each muscle were normalized to the maximum averaged evoked response in both electrode configurations (one normalization for conventional tSCS and one normalization across the six round electrodes). Data was normalized separately for each condition since the maximum attainable response amplitude for each muscle was determined by either saturation or discomfort at that electrode configuration. To validate the robustness of our results to the normalization process, data was re-analyzed with one normalization across all conditions.
2.3.1. Selectivity index
To quantify the degree of muscle recruitment selectivity enabled by each electrode position, we first generated a recruitment curve for each muscle. This curve represented the muscle's peak-to-peak response as a function of stimulation amplitude. We then quantified the recruitment for each muscle as the area under the curve (AUC) of its recruitment curve normalized to the maximum possible recruitment i.e. maximal recruitment at all stimulation amplitudes. Last, we computed the selectivity SI of muscle m as the normalized recruitment RECm of that muscle minus the average normalized recruitment of M—1 muscles, excluding the considered muscle, m (figure 2(E)). Conceptually, the selectivity index reflects the normalized recruitment of a given muscle compared to the average activation of all other muscles (Badi et al 2021):
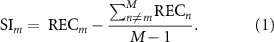
Figure 2. Muscle recruitment and selectivity by conventional and multielectrode tSCS. (A) Overlaid EMG responses are shown over a broad range of SCS amplitudes for the conventional electrode in a representative participant. Stimulation artifacts have been blanked for illustration purposes. (B) Overlaid EMG responses for the right caudal electrode in the same participant. (C) The EMG responses were averaged across n= 4 repetitions for each stimulation amplitude. The peak-to-peak amplitude was calculated to create a recruitment curve for each recorded leg muscle (color traces: targeted muscles). (D) Recruitment curves for muscle responses to stimulation by the right caudal electrode. (E) Illustration of selectivity index computation as the relative recruitment of a given muscle compared to all other muscles (for details, see section 2). (F) Selectivity index for all recorded muscles in conventional and right caudal electrode tSCS in this participant. RF: rectus femoris; VL: vastus lateralis; ST: semitendinosus; TA: tibialis anterior; MG: medial gastrocnemius; SL: soleus; R: right leg; L: left leg. Modified from (Bryson et al 2023).
Download figure:
Standard image High-resolution imageThe selectivity index varies from −1 to 1 and reflects the activation of a targeted muscle compared to the average activation of all other muscles, with a value of 0 indicating that a given muscle is recruited equally to the average of all other muscles. This computation was performed for each muscle, electrode configuration, and participant.
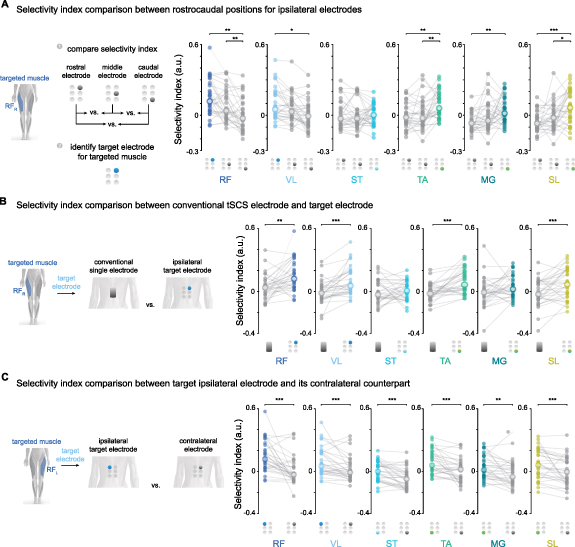
To identify the target electrode for each muscle, we first compared the selectivity index enabled by three electrode positions ipsilateral to the target muscle (rostral: T10/T11, middle: T11/T12, caudal: T12/L1) and selected the electrode with the highest median selectivity index as the target electrode for that muscle. We then tested whether this target electrode enabled a significantly higher selectivity index for the targeted muscle compared to the conventional tSCS electrode and whether the selectivity index by the target electrode was higher than by its contralateral counterpart i.e. the small-diameter electrode at the same rostrocaudal level on the contralateral side.
2.3.2. Paired pulse suppression
The amount of suppression by post-activation depression was calculated as the ratio between the second (R2) to the first (R1) response amplitudes. An R2/R1 ratio lower than 1 indicates that the amplitude of the evoked response to the second pulse was lower than the amplitude of the first response. The amount of suppression has been shown to be inversely related to the stimulation amplitude (Danner et al 2016, Skiadopoulos et al 2022), so we quantified suppression at the highest stimulation amplitude, where the amount of suppression should be the lowest. This choice was deliberate to facilitate a conservative analysis of recruitment mechanisms in a 'worst-case' scenario.
2.3.3. Recruitment probability and spinal activation maps
The probability of recruiting a given muscle was computed for each electrode configuration as the normalized AUC of its recruitment curve at that electrode position. AUC values for each muscle were normalized to the AUC in the electrode that achieved the highest level of recruitment. We then computed the recruitment probability for each muscle as the average normalized recruitment across participants. In this computation, a recruitment probability of 1 would indicate that that muscle was maximally recruited at that electrode position for all participants.
Recruitment probability estimates were used to model the activation of motoneuron pools in each spinal segment Si as a linear combination of the normalized leg muscle recruitment Mj, and Wi,j , the expected segmental distributions of motoneuron pools innervating muscle j at spinal segment Si, (Wagner et al 2018):
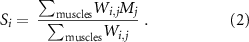
The coefficients Wi,j were obtained from an innervation probability map constructed by Hofstoetter et al, which included qualitative and quantitative data from thousands of participants in anatomical textbooks and electrophysiological studies (Hofstoetter et al 2021b). Probability estimates for the VL muscle were derived from a separate anatomical study showing similar innervation in quadriceps muscles within the L2-L4 spinal segments (Sharrard 1964, Wagner et al 2018). The resulting spinal activation values were interpolated and superimposed onto a 2D image of the human lumbosacral spinal cord.
2.4. Statistics
Statistical analyses were performed using the SciPy statistics toolbox (v1.8.1) for Python. Data for left and right leg muscles were pooled together for analysis. Normal distribution was tested using the Kolmogorov-Smirnov test. As a great majority of datasets were not normally distributed, non-parametric statistical tests were used. A Friedman test for repeated measures was performed to determine the effect of the multielectrode rostrocaudal vertebral level (rostral, middle, and caudal) on selectivity index. A Wilcoxon signed-rank test with a Bonferroni correction for multiple comparisons was then used to determine significant differences in selectivity index across electrode positions. Separate Wilcoxon signed-rank tests were used to compare the selectivity index enabled by the target electrode against the conventional tSCS, as well as against its contralateral counterpart. A Wilcoxon rank sum test was used to test for significant suppression in each muscle by its target electrode.
3. Results
3.1. Multielectrode configuration of tSCS results in enhanced rostrocaudal and unilateral selectivity compared to conventional tSCS
To study muscle recruitment enabled by conventional and multielectrode configuration electrodes, we recorded leg muscle responses over a broad range of stimulation amplitudes. The motor thresholds for the conventional and multielectrode configurations are shown in supplementary table 2. Overlaid EMG responses to the first stimulation pulse at increasing amplitudes for the conventional and right caudal electrodes are shown for a representative participant in figures 2(A) and (B). Notably, response latencies were progressively higher for caudally innervated muscles in both electrode configurations. This increase in latency is in agreement with previous observations (Courtine et al 2007, Minassian et al 2007a), suggesting that the increase in latency is due to the distance between motor neuron pools and the innervated muscle rather than the electrode location. Identification of the nature of the responses based on their latencies is not always definitive (Ertekin et al 1996, Troni et al 1996). Therefore, we applied existing neurophysiological methods to further analyze these responses.
To understand the relationship between muscle recruitment and stimulation amplitude, we calculated the peak-to-peak amplitude of the averaged responses for each recorded leg muscle (figures 2(C) and (D)). The degree of muscle recruitment was proportional to the stimulation amplitude. However, the degree of recruitment for a given muscle at a given amplitude differed across electrode configurations. Stimulation by the conventional tSCS electrode configuration resulted in broad non-selective muscle recruitment, as reflected by similar motor thresholds across rostral, caudal, and bilateral muscles (figure 2(C)). In contrast, motor thresholds were lower in a subset of muscles recruited by the right caudal electrode, and their recruitment level was close to saturation by the motor threshold amplitude of the others (figure 2(D), note the differences in recruitment level at ∼40 mA). The left leg distal muscles (TA, MG, SL) were also recruited at low amplitudes by the right caudal electrode in this participant. A common observation was that the targeted recruitment of a particular muscle group by the multielectrode configuration was usually accompanied by some undesired recruitment of the other unilateral muscle group (recruitment of right leg proximal muscles in the targeting of right leg distal muscles or vice versa), or the contralateral muscle group, as in this example. Therefore, we sought to quantify recruitment selectivity across muscles and electrode configurations.
To better interpret the degree of muscle recruitment selectivity enabled by each electrode position, we computed the selectivity index (Raspopovic et al 2011, Badi et al 2021) for all muscles (figure 2(E)). Selectivity indices for the conventional and right caudal electrodes in this participant are shown in figure 2(F). The conventional tSCS electrode primarily enabled the recruitment of bilateral proximal muscles (RF, ST, VL), as indicated by a positive selectivity index. The AUC of the recruitment curve for distal muscles (TA, MG, SL) was lower than all others, resulting in a low and negative selectivity index for these muscles. In contrast, for the multielectrode configuration, the selectivity indices of distal muscles of the targeted (right) and non-targeted (left) legs were higher than most other muscles. Moreover, the relative activation of proximal muscles in both legs was lower than that for all other muscles, resulting in a low selectivity index for the non-targeted proximal muscles in this participant. To understand trends in recruitment selectivity that were common across participants, we performed group-level analyses.
3.2. Rostrocaudal and ipsilateral positioning over muscle-specific motor neuron pools enhances recruitment selectivity of targeted muscle groups
To identify the optimal electrode position to target each recorded leg muscle, we first computed the selectivity index for all muscles and electrode configurations across participants. We then compared the selectivity index enabled by each electrode rostrocaudal position (rostral, middle, or caudal) ipsilateral to the targeted muscle (figure 3(A)). Selectivity indices enabled by the multielectrode configuration depended on an electrode's rostrocaudal position for most recorded muscles: Friedman effect of electrode's rostrocaudal position in RF (χ2 = 9.75, p = 0.008), VL (χ2 = 15.06, p < 0.001), ST (χ2 = 0.063, p = 0.969), TA (χ2 = 10.94, p = 0.004), MG (χ2 = 6.06, p = 0.048), and SL (χ2 = 10.56, p = 0.005). Statistical significance for comparisons across electrode positions is shown above each muscle. The electrode with the highest median selectivity index was taken as the target electrode for that muscle for all subsequent analyses. Results for normality using the KolmogorovSmirnov test can be found in supplementary table 3.
Figure 3. Selectivity index comparison between electrode types and positions. (A) Selectivity index comparison between electrodes in three rostrocaudal positions ipsilateral to the targeted muscle. The electrode with the highest median selectivity index is illustrated in color and was chosen as the target electrode for that muscle. Muscles from both legs are pooled together, with two data points per participant (e.g. one data point for the right RF targeted by the right rostral electrode and one data point for the left RF targeted by the left rostral electrode). (B) Selectivity index comparison between the target ipsilateral electrode and the conventional tSCS electrode. (C) Selectivity index comparison between the targeted ipsilateral electrode and its contralateral counterpart. Selectivity indices for most muscles were higher for electrodes ipsilateral to the targeted muscle than those achieved by the contralateral electrode. RF: rectus femoris; VL: vastus lateralis; ST: semitendinosus; TA: tibialis anterior; MG: medial gastrocnemius; SL: soleus; R: right leg; L: left leg. The central white circles with colored outlines are the median. Friedman test for repeated measures for the effect of electrode position on selectivity index followed. Wilcoxon signed rank test with Bonferroni correction for multiple comparisons for comparisons across electrode positions. * p < 0.05; ** p ⩽ 0.01; *** p ⩽ 0.001. Modified from (Bryson et al 2023).
Download figure:
Standard image High-resolution imageWe hypothesized that the target ipsilateral electrode in the multielectrode configuration could enhance the recruitment selectivity of a targeted muscle group compared to conventional tSCS (figure 1(D)). To test this hypothesis, we compared the selectivity index for each muscle enabled by the conventional tSCS and the ipsilateral target electrode (figure 3(B)). Overall, the target electrode in the multielectrode configuration significantly enhanced recruitment selectivity compared to the conventional electrode for most muscles: Wilcoxon signed rank effect of RF (W =99, p = 0.002), VL (W =43, p < 0.001), ST (W =228, p = 0.500), TA (W =54, p< 0.001), MG (W =175, p = 0.096), and SL (W =94, p = 0.001).
To test whether the improvements in muscle recruitment selectivity could be achieved by having the small diameter electrode at the right rostrocaudal level regardless of the electrode's lateral position, we compared the selectivity index between the target ipsilateral electrode and its contralateral counterpart (figure 3(C)). As an example, we compared the selectivity index of the right RF when targeted by the right rostral electrode to the selectivity index of the same muscle targeted by the left rostral electrode. Overall, the ipsilateral target electrode significantly enhanced recruitment selectivity compared to its contralateral counterpart: Wilcoxon signed rank effect of RF (W =54, p < 0.001), VL (W =80, p < 0.001), ST (W =55, p < 0.001), TA (W =75, p< 0.001), MG (W =100, p = 0.002), and SL (W =65, p < 0.001).
Although selectivity index values for individual participants slightly varied when normalization of peak responses was performed across all electrode configurations (supplementary figure 1), the general trend across electrodes remained the same. The target electrode in the multielectrode configuration was the same for each muscle and achieved a higher level of selectivity than the conventional electrode and its contralateral counterpart. These results indicate that positioning small-diameter surface electrodes over the predicted locations of a muscle's motoneuron pools in a rostrocaudal direction while maintaining laterality can improve the recruitment selectivity of that muscle.
3.3. Elicited responses with multielectrode configurations are mediated by posterior root-muscle reflexes
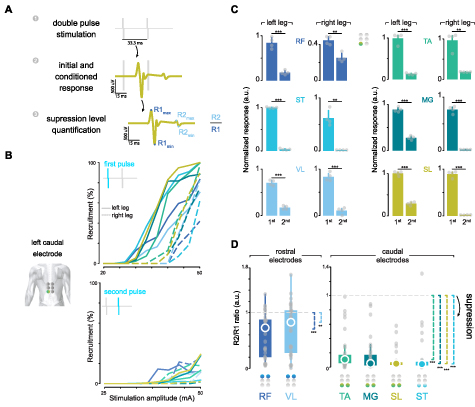
Studies examining the potential use of tSCS for recovery of motor function below the injury use the posterior root-muscle (PRM) reflex to confirm the effective position of the stimulating electrode over the spinal cord (Hofstoetter et al 2015, Minassian et al 2016, Gad et al 2017, Sayenko et al 2019). To investigate the reflex mechanisms behind spatially selective tSCS, we included in the recruitment curves protocol an additional paired pulse with a conditioning-test interval of 33.3 ms (figure 4(A)). We hypothesized that if elicited responses were indeed mediated by PRM reflexes, compound muscle action potentials to the test (second) pulse would reflect significant depression, a hallmark behavior of reflex responses (Olsen and Diamantopoulos 1967, Hofstoetter et al 2019).
Figure 4. Verification of afferent stimulation by posterior root-muscle reflexes. (A) Exemplary paired-pulse response in SL muscle. We verified that small-diameter electrodes in the multielectrode configuration recruited primarily afferent fibers by the suppression of posterior-root muscle reflexes using a paired-pulse paradigm with an inter-pulse interval of 33.3 ms. The amount of suppression was quantified by the response amplitude ratio between the responses to the first and the second stimulation pulses. (B) Recruitment curves in a representative participant for the first and second pulse responses in the left caudal electrode from the multielectrode configuration. (C) First and second peak-to-peak responses at the highest stimulation amplitude in the same participant as in panel (B). Peak responses are normalized to the highest muscle response across the multielectrode configuration. (D) Group analysis of R2–R1 amplitude ratio across participants and electrodes. The right and left legs are pooled together. A value of 1 would indicate that the first and second responses had equal magnitudes, whereas a value <1 would indicate that the response to the second stimulation pulse was lower than the one to the first pulse. The colored circle in the array indicates the optimal electrode for each muscle. In panel (C), bars represent the mean ± s.d. Paired-sample t-test. In panel (D), the central circle is the median, while the top and bottom edges represent the 75th to 25th percentile, respectively. One-sample Wilcoxon signed rank test with the alternative hypothesis that μ–1 < 0. * p < 0.05; ** p ⩽ 0.01; *** p ⩽ 0.001. RF: rectus femoris; VL: vastus lateralis; ST: semitendinosus; TA: tibialis anterior; MG: medial gastrocnemius; SL: soleus; R: right leg; L: left leg. Modified from (Bryson et al 2023).
Download figure:
Standard image High-resolution imageRecruitment curves for the first (top panel) and second (bottom panel) pulses delivered through the left rostrocaudal electrode in a representative participant are shown in figure 4(B). The amplitude of the response to the second pulse was largely suppressed in most muscles. However, the amount of suppression was inversely proportional to stimulation amplitude. Therefore, we quantified the level of suppression for each electrode at the highest stimulation amplitude, where the amount of suppression was generally the lowest. A comparison in response amplitudes between the first and the second pulse for the left caudal electrode in the same representative participant is shown in figure 4(C). Overall, there was a significant reduction in response amplitude to the second stimulation by the left caudal electrode in that participant.
To analyze this effect across participants, we computed the level of suppression as the ratio between the first and second responses. A ratio smaller than one would indicate response suppression, i.e. that the response to the second stimulation pulse was lower than the response to the first pulse. The amount of suppression for each muscle, when targeted by its optimal electrode, is shown in figure 4(D). There was a significant amount of post-activation depression of the evoked response by the paired-pulse paradigm at each optimal electrode configuration: one-sided 1-sample Wilcoxon signed rank test in RF (W =489, p < 0.001), VL (W =407, p = 0.004), ST (W =527, p < 0.001), TA (W =528, p < 0.001), MG (W =528, p < 0.001), and SL (W =528, p < 0.001). The amount of suppression for all electrode configurations and muscles is shown in supplementary figure 2. A significant amount of suppression was also observed in the conventional tSCS electrode and has been extensively reported by several groups (Hofstoetter et al 2019, 2021a, Oh et al 2022, Dalrymple et al 2023). These results serve as a neurophysiological confirmation that the optimal multielectrode configuration enables the effective stimulation of the respective segmental afferents in the targeted leg muscles.
3.4. Recruitment probability for each muscle reflects the segmental distribution of its motor neuron pools in the spinal cord
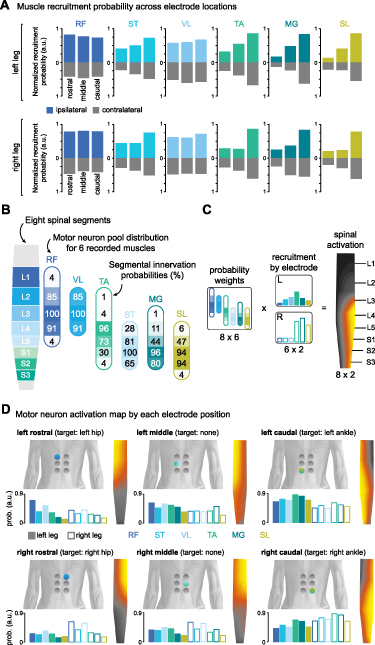
To understand how different electrode configurations could target the posterior roots projecting to spinal cord segments containing the motor neurons responsible for activating the hip, knee, and ankle joints, we developed a neuroanatomical map of spinal cord activation for each electrode position. We first calculated the probability of activating each muscle at each electrode position by averaging the normalized recruitment of that muscle across participants (see section 2). Recruitment probabilities for electrodes located ipsilaterally and contralaterally to the targeted muscles at different rostrocaudal positions are shown in figure 5(A). Next, we compiled an atlas of the expected anatomical locations for the motor neuron pools associated with the recorded leg muscles (Sharrard 1964, Wagner et al 2018, Hofstoetter et al 2021b) (figure 5(B)). Finally, we projected the recruitment probability of each muscle onto its respective innervation probabilities at each spinal segment (figure 5(C)). The resulting spinal activation maps elicited by each multielectrode position are shown in figure 5(D).
Figure 5. Neuroanatomical activation map by muscle recruitment probability. (A) Muscle recruitment probability for each muscle when the active electrode is at different rostrocaudal levels ipsilaterally (color) and contralaterally (grey) to the recorded muscle. Data were derived from the probability of recruiting a given muscle at a specific electrode position across all participants, where a probability of 1 would indicate that that muscle was maximally recruited for all participants at that electrode position. Note that values for recruitment probability by contralateral electrodes are also positive. (B) Segmental innervation probabilities for recorded leg muscles at different spinal segments. Innervation probabilities are reflected by percentage and opacity. Modified from (Sharrard 1964, Wagner et al 2018, Hofstoetter et al 2021b). (C) Computation of spinal activation maps as a linear combination of the normalized recruitment of each muscle and its innervation probability at each segment. (D) Recruitment probabilities and motor neuron activation map enabled by each electrode configuration. RF: rectus femoris; VL: vastus lateralis; ST: semitendinosus; TA: tibialis anterior; MG: medial gastrocnemius; SL: soleus; R: right leg; L: left leg. Modified from (Bryson et al 2023).
Download figure:
Standard image High-resolution imageOur analysis of recruitment probability across participants revealed a high similarity between the optimal electrode configurations and the recruitment of the posterior roots projecting to the targeted spinal cord regions involved in the activation of the hip and ankle joints. For example, the left rostrocaudal electrode predominantly activated upper left lumbar segments (figure 5(D), top left panel), while the right caudal electrode activated motor neuron pools located around right sacral segments (figure 5(D), bottom right panel). It is important to note that the combination of the segmental innervation probabilities and the muscle recruitment probability was agnostic to the electrode configuration. Nevertheless, the spinal activation maps closely reflect the cathode location for that configuration.
4. Discussion
In this work, we present a technological framework to evaluate and quantify muscle recruitment selectivity enabled by different electrode configurations of tSCS. We identify optimal cathode positions to target different leg muscles by computing the selectivity index across 12 muscles and electrode configurations in 16 participants, with a total of 192 analyzed muscles. We demonstrate that a small-diameter multielectrode configuration of tSCS over the T10-L1 vertebral segments can enhance the rostrocaudal and unilateral recruitment selectivity of leg muscles compared to conventional tSCS using a single large electrode over the T11-T12 vertebra. Verification of post-activation depression suggests that these improvements in muscle recruitment selectivity are mediated by posterior root-muscle reflexes, which is a promising finding for the translation of this technology into clinical practice. Here, we discuss our findings and their implications in neurorehabilitation for people with neuromotor disorders.
4.1. Improving spatial selectivity by non-invasive technologies
Our study builds upon previous research in non-invasive tSCS, which has shown that stimulating different areas of the lumbar spinal cord can selectively activate motor neuron pools in leg muscles. Specifically, rostral and caudal positions of small electrodes centered over the midline have been shown to activate proximal and distal muscle groups, respectively (Krenn et al 2015, Sayenko et al 2015), and lateral positions can selectively recruit both proximal and distal muscles ipsilateral to the cathode position (Calvert et al 2019).
In our work, we demonstrate that by combining these approaches, we can achieve selective activation of specific muscle groups in the hip, knee, and ankle joints, independently for the right and left legs. We found that different rostrocaudal positions of a small diameter electrode provided unique degrees of selectivity for different muscles. The rostral electrode, positioned lateral to the midline and centered rostrocaudally over the T10/T11 interspinous ligament, was the optimal position for RF and VL muscles, whereas the caudal electrode, centered over the T12/L1 ligament, was the optimal contact for TA, MG, and SL. However, optimal selectivity for the ST was more challenging to achieve. The caudal electrode was marginally better than other rostrocaudal positions, but this difference was non-significant. Despite the caudal segment being the most probable site for ST recruitment, we observed that ST muscle recruitment was highly likely to occur at low stimulation amplitudes across all three segments. This consistent recruitment of ST and other hamstring muscles at different stimulation sites has been previously observed by other groups and has been attributed to their broader segmental innervation than other muscles (Hofstoetter et al 2021b). In their work, Hofstoetter and colleagues argue that PRM reflexes of the hamstring muscles may not provide useful information in intraoperative monitoring to guide electrode placement. Our results similarly suggest that responses from the other leg muscles should be prioritized when selecting the optimal electrode placement for a particular individual.
Interestingly, we found that the middle electrode, which was centered at the T11/T12 vertebral level, did not provide optimal selectivity for any muscle. This finding is noteworthy because conventional tSCS protocols often center the electrode at this level. We do not suggest that this placement is incorrect or ineffective, as if the objective were to target both proximal and distal muscles simultaneously by one stimulation site, T11/T12 or T12/L1 would be ideal placements. Rather, it may not be the optimal position for achieving selective activation of specific muscle groups.
Despite this, we do not recommend discarding the T11/T12 position in the multielectrode configuration altogether. In fact, it may be crucial in the context of multipolar configurations of tSCS, which have been shown to enhance recruitment selectivity further and prevent the unwanted activation of non-targeted muscles in eSCS (Struijk et al 1993, Wagner et al 2018, Hofstoetter et al 2021b, Rowald et al 2022). Therefore, while T11/T12 may not be the most selective electrode position for targeting specific muscles, it may still have important roles in optimizing tSCS protocols for further personalized improvements.
4.2. Toward personalized improvements in muscle recruitment
Our study revealed that achieving optimal recruitment selectivity using tSCS is not universal across individuals and targeted muscles. As shown in figure 3(B), the conventional tSCS electrode placement outperformed the target electrode in some participants' muscles. While our results provide a valuable starting point for improving muscle recruitment selectivity in individuals with neuromotor disorders, it is important to recognize that interindividual differences in neuroanatomy and neurophysiology, particularly in the damaged nervous system, will require personalized approaches. To optimize stimulation protocols for each individual's residual ability and specific needs, we believe it is crucial to test muscle recruitment for each participant and verify that these activations are mediated by posterior root-muscle reflexes. Modifications in stimulation parameters such as amplitude, frequency, and pulse width should then be made and reported based on these initial activation thresholds and selectivity starting points.
Recent advancements in eSCS have demonstrated the potential of machine learning approaches to identify optimal stimulation parameters for targeting motor function rapidly, across subjects and species (Govindarajan et al 2022, Bonizzato et al 2023). Similarly, algorithms for automated calibration of stimulation parameters can be employed to determine clinically suitable tSCS settings across participants with different anatomies and neuromotor disorders (Salchow-Hömmen et al 2021). Therefore, these approaches hold promise for streamlining and optimizing the customization of stimulation parameters in tSCS applications.
4.3. Importance of verification of effective stimulation sites by PRM reflex
The motor-enabling effects of SCS are primarily associated with the recruitment of proprioceptive afferent fibers in the posterior roots (Dimitrijevic et al 1980, Capogrosso et al 2013, Minassian et al 2016, Formento et al 2018). However, because of the complex topographic anatomy in the lumbosacral spinal cord (Wall et al 1990), non-invasive stimulation at different lumbosacral vertebral segments can excite both afferent and efferent pathways to different degrees. The engagement of each pathway is crucial to both the immediate prosthetic effect that may be enabled by tSCS and the rehabilitation effect that may be observed through SCS-assisted therapy (Seáñez et al 2022). Therefore, careful evaluation of evoked responses should be performed.
While direct recruitment of motor axons within the anterior roots, such as in functional electrical stimulation, can indeed produce desired movements in leg muscles Faghri et al 1992, Popovic et al 2011), the artificial recruitment of large-diameter motor fibers makes it technically challenging to produce and sustain large forces (Kirsch and Rymer 1987, Giat et al 1993). Moreover, the post-synaptic activation of motor fibers bypassing spinal and descending circuits limits the types of movements that can be performed to those that can be pre-programmed (Ajiboye et al 2017), and prevents the voluntary modulation and neuroplasticity potential of these prosthetic effects. In contrast, the recruitment of afferent fibers by SCS enables interaction with descending and spinal circuits, which can enhance residual descending inputs (Minassian et al 2016, Guiho et al 2021) and lead to a natural recruitment order that is fatigue-resistant and capable of sustaining the whole body weight for extended periods (Formento et al 2018, Wagner et al 2018). This presynaptic recruitment of primary afferents, in turn, promotes neuroplasticity, which is believed to mediate the neurological recovery observed during neurorehabilitation facilitated by SCS (Nishimura et al 2013, Asboth et al 2018).
Overall, evoked responses by all target electrodes were mediated by PRM reflexes, as evidenced by the suppression of the conditioned response. However, the probability of efferent fiber recruitment differed substantially between L2-L4 innervated (RF, and VL) and L4-S2 innervated (ST, MG, TA, and SL) muscles (figure 4(D)). There were a few cases for proximal muscles in which the response average amplitude to the second pulse was higher than the average amplitude of the first response (values >1 in RF, ST, and VL). This observation has been recently reported by others (Oh et al 2022, Skiadopoulos et al 2022), although, to our knowledge, has not been studied in detail. In contrast, there were no cases suggesting efferent recruitment in TA, MG, or SL, and only one case in ST. This suggests that higher stimulation amplitudes could be employed at caudal segments to target distal muscles with a reduced likelihood of recruiting motoneurons directly.
It should be noted that the degree of suppression was calculated at the maximum stimulation amplitude, which is associated with a greater likelihood of directly recruiting motor neurons. This selection was deliberate to facilitate a conservative analysis of recruitment mechanisms in a 'worst-case' scenario. Routine applications of tSCS use stimulation amplitudes between 0.8 and 1.2 times the motor threshold to enhance motor function after paralysis (Hofstoetter et al 2013, Minassian et al 2016, Inanici et al 2021, Samejima et al 2022). As these amplitudes are considerably lower than saturation, we expect the probability of directly recruiting anterior roots in a therapy setting to be lower than those reported here. Nevertheless, because spine curvature can dramatically alter the probability of directly recruiting the anterior roots (Binder et al 2021), verification of post-activation depression should be carefully confirmed in different therapeutic settings (e.g. sitting, standing, or in the position required to use a rehabilitation device).
4.4. Potential of improved selectivity in tSCS for neurorehabilitation
Despite the marked effects of stimulation frequency, intensity, and sensory feedback on evoked responses (Sayenko et al 2014, 2015), continuous SCS is commonly used in studies involving SCS-assisted neurorehabilitation (Gerasimenko et al 2015a, Meyer et al 2020, Inanici et al 2021). In this application, stimulation parameters are typically fixed at the onset of therapy and kept constant across different types of movements. This continuous, non-selective SCS largely diverges from the natural spatiotemporal activation patterns observed during movement (Yakovenko et al 2002) and can disrupt the natural feedback from proprioceptive afferents (Formento et al 2018), which is critical to enable the spinal regulation of movements during fine motor control (Barra et al 2022). And although long-term training combined with continuous SCS can indeed induce improvements in motor function that persist even without stimulation, they may take several months to a year of intense rehabilitation to appear (Angeli et al 2018, Gill et al 2018, Seáñez and Capogrosso 2021).
Spatial and temporal control of SCS aims to facilitate movements through the selective activation of specific motor neuron pools at the appropriate phases of movement (Wenger et al 2014, 2016). Recent clinical studies have shown that spatiotemporal eSCS can rapidly enable (within one week) powerful facilitation of walking, cycling, kayaking, and other dexterous activities in people with SCI (Wagner et al 2018, Rowald et al 2022) as well as upper limb movements and grasping ability in people with stroke (Powell et al 2023).
Combining long-term activity-based training with tSCS has been shown to improve standing, balance, and induce functional recovery (Gerasimenko et al 2015a, Gad et al 2017, Sayenko et al 2019) that is comparable to that achieved through eSCS (Harkema et al 2011, Rejc et al 2015, Seáñez and Capogrosso 2021). It is unlikely that non-invasive tSCS will achieve the same level of muscle recruitment selectivity as eSCS. However, using multielectrode configurations of tSCS to selectively target facilitation in certain muscles during movements could help individuals learn to perform complex movements faster, potentially accelerating their recovery process. By speeding up the recovery process, these technologies could then become more accessible to individuals with SCI by reducing the amount of training required and, ultimately, the cost.
4.5. Study limitations and implications for future studies
4.5.1. Selective targeting of agonist and antagonist muscles
In this work, we demonstrate that we can target proximal vs. caudal as well as ipsilateral vs. contralateral muscles from one multielectrode configuration. However, it is important to note that the electrode that results in optimal selectivity for a given muscle is the same electrode that results in optimal selectivity for its antagonist muscle, e.g. the bottom, ipsilateral electrode, is the optimal one for TA and MG/SL (figure 3). This result may be partially attributable to the significant overlap in locations of posterior roots for both flexor and extensor muscles in the lumbosacral spinal cord (Sharrard 1964, Schirmer et al 2011, Hofstoetter et al 2021b, Rowald et al 2022). However, even though posterior roots for biceps and triceps muscles do not have the same level of overlap in the cervical spinal cord (Schirmer et al 2011, Greiner et al 2021), both muscles are generally recruited to the same degree at different rostrocaudal stimulation locations in multielectrode arrays for cervical tSCS (de Freitas et al 2021, Oh et al 2022). Therefore, this may also be a limitation of the amount of selectivity that can be achieved non-invasively and can present significant challenges in applying these technologies to functional tasks such as locomotion. However, different methods may be explored in future studies to further enhance selectivity.
4.5.2. Potential improvements by different electrode configurations and higher electrode density
The invasive nature of eSCS offers advantages in terms of electrode placement and proximity to the spinal cord, enabling more precise targeting of specific neural structures and resulting in enhanced selectivity compared to tSCS. While direct comparative studies are necessary for a comprehensive analysis of selectivity differences between the two techniques, previous findings allow us to illustrate some similarities and distinctions between both approaches. In both eSCS and tSCS of the lumbosacral spinal cord, agonists and antagonist muscles are recruited simultaneously when targeting proximal or distal muscle groups (Wagner et al 2018). However, unlike in tSCS, eSCS enables proximal muscles to be selectively targeted without the recruitment of distal muscles, and vice versa.
In eSCS, various stimulation patterns have been explored to address the challenge of overlapping agonist and antagonist posterior roots. For instance, in multipolar electrode configurations, electrodes surrounding the cathode electrode are simultaneously stimulated as anodes. This approach is thought to help shield the distribution of the cathodic field and limit the unintended activation of the non-targeted muscles (Wagner et al 2018, Hofstoetter et al 2021b, Rowald et al 2022, Lorach et al 2023). However, it is important to note that, to our knowledge, this approach has not been specifically tested in tSCS, where the return electrode would still be positioned on the anterior side of the body. Therefore, thorough testing is required to evaluate its effectiveness in the tSCS context.
In this work, we aimed to improve selectivity in 12 muscles by using an electrode grid of only six electrodes, and only four of those resulted in optimal selectivity for some muscles. Theoretically, increasing the number of electrodes in multielectrode arrays with small-diameter electrodes could further improve recruitment selectivity (Oh et al 2022). However, in the clinical application of this approach, where continuous 30 Hz stimulation may be delivered, it is crucial to consider the safety implications of an increase in power density. In this study, the recommended maximum power density for the Axelgaard electrodes used was not to exceed 0.1 W cm−2.
Another important consideration in the use of smaller electrodes is that the higher charge density resulting from a smaller surface area may make it challenging to stimulate at effective amplitudes near the motor threshold without causing discomfort. Although recent work using the same diameter electrodes has reported participants being able to tolerate stimulation amplitudes up to 200 mA during single pulses (Manson et al 2020), tolerance may be lower during continuous 30 Hz stimulation necessary for therapy. The use of high-frequency carriers for burst stimulation has been shown to enable higher stimulation amplitudes than conventional tSCS without causing discomfort (Gerasimenko et al 2015a, Gad et al 2017, Inanici et al 2021). However, recent work has shown no differences in comfort levels when these are normalized to elicit motor responses of equal magnitudes as conventional, unmodulated waveforms (Manson et al 2020, Dalrymple et al 2023). Therefore, tolerance to stimulation remains a critical barrier to the clinical translation of tSCS that will require further research.
4.5.3. Robustness across experimental sessions
We show that muscle recruitment selectivity is higher in multielectrode configurations than in conventional tSCS when tested within the same session. However, in the context of rehabilitation, daily positioning of surface electrodes is necessary, making it impractical to test selectivity or PRM suppression on a daily basis. Therefore, it is important to investigate the consistency of our findings within and across participants when tested on different days. It remains unknown whether the optimal position for achieving selectivity in a particular muscle during one session will achieve the same level of selectivity when tested in a subsequent session. This is an important consideration for the development of effective and efficient tSCS-based rehabilitation protocols.
4.5.4. Translation into clinical applications
While our study demonstrated improved selectivity using single pulse responses, translating these findings into continuous muscle activation patterns required for movement poses significant challenges. During continuous stimulation, we may encounter difficulties in overcoming antidromic collisions between stimulation pulses and proprioceptive feedback, which can hinder movement ability (Formento et al 2018). Stimulation tolerance is another important factor that may impact patient adherence and participant withdrawal (Moon et al 2021). Further research is needed to investigate the neural mechanisms underlying muscle recruitment selectivity during continuous stimulation and to determine the optimal balance between efficacy and tolerability in tSCS.
4.5.5. Controls vs SCI
Our study was conducted on neurologically intact individuals, while the intended population for this technology consists of individuals with SCI. An important consideration is that achieving selective stimulation in muscles with significant atrophy in people with SCI may pose a significant challenge. To enable selective stimulation in the atrophied muscle, we may require higher stimulation amplitudes that either cause discomfort or recruit non-targeted muscles before targeted ones. Therefore, it is essential to validate these findings in a population of individuals with SCI to ensure the feasibility of the technology in the intended population.
5. Conclusions
In summary, we demonstrate that the use of multielectrode tSCS results in enhanced rostrocaudal and unilateral selectivity compared to conventional tSCS. The optimal electrode position for targeting each leg muscle depends on the segmental innervation of its motor neuron pools within the spinal cord and allows for the targeted recruitment of specific muscle groups while minimizing the recruitment of other non-targeted muscle groups. As these are PRM-reflex-mediated responses, the interaction with descending and spinal neural circuits may enable the translation of this technology into effective therapies that aim to improve movement capacity in people with SCI.
Data availability statement
The data cannot be made publicly available upon publication because they are not available in a format that is sufficiently accessible or reusable by other researchers. The data that support the findings of this study are available upon reasonable request from the authors.
Author contributions
N B and J D P real-time software platform development.
N B, I S, and J D P, technological framework.
N B, R H, J F, L L, R K, and I S performed experiments.
N B, R H, J F, L L, and R K, and I S data curation, validation, and interpretation.
N B and I S formal analysis.
I S wrote original draft with N B
N B, R H, J F, L L, R K, and I S, reviewing and editing.
I S study conceptualization, supervision, and securing funding.
Conflict of interest
The authors declare no conflicts of interest in relation to this work.
Ethical statement
This study was reviewed and approved by Washington University in St. Louis Institutional Review Board, Approval Number #202105168. Research was conducted in accordance with the principles embodied in the Declaration of Helsinki and in accordance with local statutory requirements. All participants gave written informed consent.
Funding source
Research reported in this publication was supported in part by the Eunice Kennedy Shiver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K12HD073945, the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number K01NS127936, and Washington University's McDonnell Center for Systems Neuroscience Small Grants Program.
Supplementary data (1.8 MB PDF)