Abstract
Therapeutic nanoparticles can be combined with different anticancer drugs to achieve a synergistic therapy and avoid the limitations of traditional medicine and thus have clinical prospects for cancer. Herein, an effective nanoplatform was developed for self-assembling AMF@DOX-Fe3+-PEG nanoparticles (ADPF NPs) via the coordination of ferric ions (Fe3+), amentoflavone (AMF), doxorubicin (DOX), and PEG-polyphenol. The ADPF NPs possessed high drug loading efficiency, good stability and dispersion in water, prolonged blood circulation, and pH-dependent release, which leading to targeted drug transport and enhanced drug accumulation in the tumor. The AMF from the ADPF NPs could inhibit the expression of the Aldo-keto reductase family 1B10 (AKR1B10) and nuclear factor-kappa B p65 (NF-κB p65), which reduced the cardiotoxicity induced by DOX and enhanced the chemotherapy efficacy. This study established a new strategy of combining drug therapy with a nanoplatform. This new strategy has a wide application prospect in clinical tumor therapy.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
Nowadays, cancer incidence is increasing rapidly, and cancer therapy is becoming a serious challenge to global human healthcare. Among various cancers, lung cancer alone had 2093 876 new cases in 2018 and 1761 007 deaths in males and females worldwide, indicating that it is still responsible for the majority of cancer cases and deaths [1]. In terms of histologic appearance, lung cancer has two main forms, namely, non-small cell lung cancer (NSCLC) with above 85% incidence and other cases with only 15% incidence (collectively called small cell lung cancer) [2]. Overall, the survival rate of NSCLC patients is poor, particularly among those at Stage III when the cancer is detected [3]. Thus, an effective therapy approach for reducing mortality among NSCLC patients must be established.
Several studies have shown that combination therapy is an applicable treatment for stage III NSCLC patients after surgical resection and that chemotherapy as a traditional approach is still the primary treatment for cancer patients in current clinical practice guidelines [4–6]. Doxorubicin (DOX), one of the standard first-line chemotherapy drugs, is widely used due to its high antitumor efficacy by inhibiting topoisomerase II and intercalating into the DNA duplex [7, 8]. However, its clinical application is curtailed by issues, including undesired side effects, drug resistance of cancer cells, short half-life, and poor targeting. In particular, DOX-induced cardiotoxicity is the major reason that reduces the drug's therapeutic index and may even cause death [9–11]. Doxorubicinol (DOXOL) that is mainly accumulated in the heart is produced by reducing C13-keto of DOX into C13-alcohol via several enzymes, including the Aldo-keto reductase family 1B10 (AKR1B10) and carbonyl reductase. It is responsible for myocardial death and drug resistance [12–15]. Research has demonstrated that AKR1B10 is overexpressed in NSCLC, resulting in the enhanced proliferation and migration of cancer cells by upregulating the expression of nuclear factor-kappa B p65 (NF-κB p65) protein involved in activating the NF-κB signaling pathway [16]. Therefore, inhibition of the activity of AKR1B10 for avoiding DOX reduction can provide an effective means to reinforce the therapeutic efficacy of DOX and relieve cardiotoxicity.
Several studies have indicated that flavones as one kind of polyphenols produced from natural plants can inhibit AKR1B10 recombinant enzymes and might be potential anti-cancer drugs for targeted therapy due to their abundant phenolic hydroxyl [17]. Amentoflavone (AMF) belonging to biflavonoids as special flavones is rich in Selaginella doederleinii, which has been a medicinal herb for various cancers since ancient times, and it could inhibit AKR1B10 and boost the anti-cancer effect of DOX, especially in cancer cells with overexpression of AKR1B10, such as NSCLC A549 cells [18–21]. Furthermore, its bioactivities are superior to those of monoflavonoids [22–24]. However, the prominent antitumor activity of AMF is limited by its poor bioavailability due to several factors, including low aqueous solubility and gastrointestinal permeability [25, 26].
To overcome these issues, various nanotechnology-based delivery systems have been developed. For instance, a solid proliposome modified by isomalto-olig osaccharides has been used to construct solid lipid nanoparticles for improving oral bioavailability of AMF [27]. Recently, another novel carrier applied with tocopheryl polyethylene glyco succinate and a polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer has been constructed for mixed micelles, which could improve oral bioavailability and anti-A549 cell efficacy of AMF [28]. Based on a pharmacokinetic investigation, the bioavailability of AMF by intravenous injection is much higher than its oral bioavailability [29]; however, the bioavailability of AMF alone or in combination with DOX by intravenous injection has not been reported.
In early 1995, the first FDA-approved nanodrug via intravenous injection is a liposome-encapsulating DOX nanomedicine (DOXIL) and manifested a unique protection against DOX-induced cardiomyopathy [30]. But a substantially increased occurrence of two serious adverse effects, which include the complement activation-related pseudoallergy and palmar plantar erythrodysesthesia, were caused by intravenous injection of DOXIL [31–33]; to date, only 5 based-liposomal anticancer drugs were approved by FDA [34]. Since the last-FDA-approved liposomal-based anticancer drugs in 2012, the low toxicity and high efficiency nanotechnology-based delivery systems is still a challenge for liposomes and nanomedicine development [35–37].
Recently, a one-step method of producing self-assembled metal-phenolic networks (MPNs) could act as a versatile platform for engineering nanomaterials with negligible cytotoxicity and pH responsiveness; especially, using the ferric ions (Fe3+) as an essential metal element in metabolism for coordination with natural polyphenols to construct the nanomaterials have the characteristic of the ease, low cost, and scalability of the assembly process [38, 39]. The above nano-technological progress provides a promising strategy for further development of MPNs as nanomedicine platform for cancer therapy [40]; for instance, a novel reactive oxygen species synergistic nanomedicine platform was established for enhancing chemotherapy by fenton reaction [41]. Subsequently, another self-assembled nanoplatform combining both DOX and polyphenols (epigallocatechin-3-o-gallate) could reduce cardiac toxicity by inhibiting DOXOL, exhibiting much improved antitumor effect over free DOX by promoting p53 [42]. Using the same method, the myricetin-based self-assembled NPs showed synergistic cancer therapy by antioxidation pathway, being worth noting that the issue relating insolubility of myricetin was solved [43]. However, no study has examined the NPs of AMF and DOX based on Fe3+.
In the current study, a self-assembled nanoplatform based on the coordinated chemistry of Fe3+ and AMF was developed to build AMF@DOX-Fe3+ NPs (ADPF NPs) via high-molecular-weight PEG. The as-prepared nanoplatform encapsulated with both DOX and AMF could maintain its targeting capability due to its advantages of prolonged blood circulation, high accumulation in the tumor site, and pH-dependent release. Furthermore, the ADPF NPs could inhibit the growth of the malignant solid tumor NSCLC A549 and reduce DOX-induced cardiotoxicity through the inhibition of AKR1B10.
Experimental
Materials and cell lines
DOX and AMF were purchased from Dalian Meilun Biotechnology Corporation. 8-Arm-polyethylene (8-arm PEG-NHS) was bought from Shanghai Yare Biotech. Anhydrous N,N-dimethylformamide (DMF), triethylamine (TEA), 3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT), and iron (Ⅲ) chloride (FeCl3) were supplied by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The acetonitrile and methanol (chromatographically pure) used for instrumental analyses were acquired from Sigma (St. Louis, MO, USA). All reagents were used without further purification.
The detection instrument for transmission electron microscopy (TEM) was provided by China Pharmaceutical University. The Malvern instrument (ZS90, United Kingdom) was supported by the Key Laboratory of Spin Electron and Nanomaterials of Anhui Higher Education Institutes. The key equipment used were as follows: confocal microscope with an inverted fluorescence microscope (IX71, Olympus, Japan), cell flow cytometer (Accuri C6, BD Biosciences, USA), and high performance liquid chromatography (HPLC) instrument with a diode array detector (Shimadzu, Japan).
HEK 293T and A549 cells were bought from Procell (Wuhan, China) and maintained in DMEM and Ham's F-12K medium, respectively. The two types of cells were cultured in a humidified atmosphere at 37 °C containing 5% CO2 by a respective medium supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin.
Preparation of ADPF NPs
Hydrophobic DOX and PEG-polyphenols were prepared with the method described by Shan et al [42]. The hydrophobic DOX solution (20 μl, 3 mg ml−1) and AMF aqueous solution (100 μl, 1 mg ml−1) were mixed, followed by the addition of the PEG-polyphenols aqueous solution (10 μl, 50 mg ml−1) into the mixture with stirring. Then, the FeCl3 aqueous solution (100 μl, 3 mg ml−1) was added dropwise to the mixture to obtain AMF@DOX-Fe3+-PEG nanoparticles (ADPF NPs). Dialysis (14 000 MWCO) was performed to purify the ADPF NPs by removing solvents and reactants. The contents of DOX and AMF were determined by HPLC. The ADPF NPs were concentrated for further experiments.
General characterization of ADPF NPs
The particle size and zeta potential of the ADPF NPs were measured through the dynamic light scattering (DLS) method by using a Malvern particle size analyzer. The surface morphology was examined by TEM. The HPLC instrument with a diode array detector on a C18 chromatographic column (Ultimate AQ-C18, 5 μm, 4.6 × 250 mm, Welch) was used for the content determination of AMF and DOX. The mobile phases were 0.1% (v/v) TFA (A) and 100% acetonitrile (B), and the flow rate was set to 1.0 ml min−1. Gradient elution was applied, and the elution program was as follows: 20%–90% B (0–50 min). The determinand was injected at a volume of 20 μl, and the detection wavelengths were 337 (AMF) and 254 nm (DOX). The standard curves were drawn to calculate the contents of AMF and DOX.
In vitro drug release and stability analyses
The in vitro drug release behaviors of AMF and DOX from ADPF NPs were measured in different phosphate buffer saline (PBS) solutions. Specifically, 3 ml of ADPF NPs (DOX: 200 μg ml−1; AMF: 290 μg ml−1) were added to dialysis cassettes (MWCO: 8000 K) and immersed in 30 ml PBS (pH 7.40 and pH 5.50) at 37 °C with a stirring speed of 300 rpm. Next, 500 μl of the solution in the system was taken out at defined time points of 0, 0.05, 0.10, 0.50, 1, 2, 4, 8, 18, 24, and 48 h to detect the released AMF and DOX via HPLC. Meanwhile, 500 μl of fresh PBS was replenished into the system.
To measure storage stability, the ADPF NPs were stored at 4 °C for eight weeks. The particle size, dispersion index (PDI), zeta potential, and drug loading of the ADPF NPs were detected at 0, 7, 14, 28, and 56 d. The clarity was also observed.
In vitro cell uptake
The fluorescein isothiocyanate (FITC) labeled ADPF NPs were used for cell uptake experiments. In brief, the A549 cells were incubated at the same concentration (DOX: 6.8 μM) for 1, 4, and 8 h. After rinsing with PBS twice, the cells were further stained with DAPI for 15 min, and the cell images were observed by confocal microscopy (40 × objective magnification). Furthermore, quantitative analysis of cell uptake was performed using flow cytometry.
In vitro toxicity of ADPF NPs
In vitro toxicity was determined through MTT assay. The HEK 293T and A549 cell lines were seeded into 96-well plates with 5 × 103/well and cultured at 37 °C and 5% CO2 for 24 h. Then, the cells were treated with AMF, DOX, AMF + DOX, and ADPF NPs of series concentration for 48 h after removing the original medium. A fresh medium (100 μl) with an MTT solution (10 μl) was re-added to each well for another 4 h of incubation. Then, the absorbance of each well was measured at 490 nm.
Apoptosis assay of ADPF NPs
The A549 cells were seeded in six-well plates with 5 × 105/well at 37 °C and with 5% CO2 for 24 h. Subsequently, DOX, AMF + DOX, and ADPF NPs were added at a DOX concentration of 6.8 μM, and the cells were treated for 24 and 48 h, respectively. Untreated cells were used as control. At the scheduled time points, the cells were washed once after removing the medium, and 500 μl of trypsin was added for digestion. Then, the cells were collected and centrifuged at 3500 r min−1 for 5 min. The supernatant was removed, and the cells were washed with PBS. Afterward, 5 μl of Helix NP green and 1 μl of Annexin V-PE from the dead cell apoptosis kit were added for 10 min under dark conditions, and a quantitative analysis of cell apoptosis was performed using flow cytometry. Simultaneously, the morphology of apoptosis was observed by confocal microscopy.
Western blot analyses
Rabbit polyclonal antibodies of AKR1B10 and NF-κB p65 were used for Western blot analyses. The A549 cells were seeded in a six-well plate with a density of 2 × 105/well and cultured at 37 °C and 5% CO2 for 24 h. Then, the cells were treated using a fresh medium with DOX (6.8 μM), AMF (11.0 μM), DOX (6.8 μM) + AMF (11.0 μM), or ADPF NPs (DOX concentration: 6.8 μM) for 48 h. After incubation, the protein expression levels of AKR1B10 and NF-κB p65 in different experimental groups were analyzed through Western blot analyses.
In vivo acute toxicity assessment of ADPF NPs
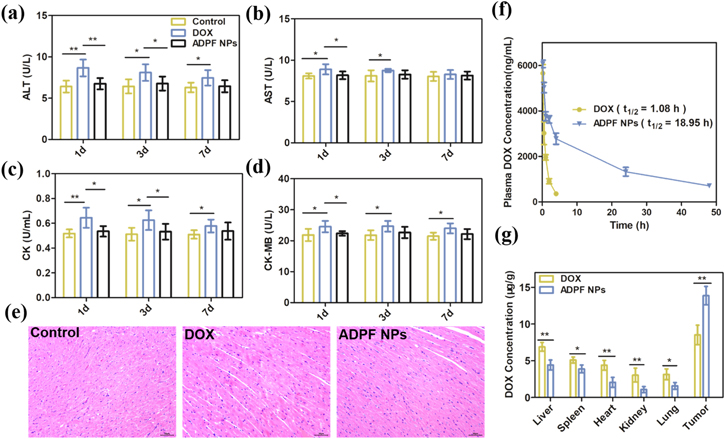
Acute toxicity assay was used to evaluate the in vivo toxicity of the ADPF NPs. A total of 12 normal BALB/C mice were randomly divided into three groups, namely, DOX, ADPF NPs, and control. Then, 200 μl of DOX and ADPF NPs containing the same DOX (DOX 2 mg kg−1) was intravenously injected into the mice. The control group was replaced with 200 μl of PBS. The experimental method was consistent with that described by Wang et al [44]. The serum biochemical parameters, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine kinase (CK), and creatine kinase-MB (CK-MB), were detected at preset time points. The heart tissues of the mice were analyzed via hematoxylin–eosin (H&E) staining after death for 7 d after administration.
Pharmacokinetic studies
Normal Balb/C mice were utilized to investigate the pharmacokinetics of the ADPF NPs in vivo. The mice were randomly divided into two groups: free DOX and ADPF NPs. All mice received 2 mg kg−1 of DOX or 2 mg kg−1 of DOX equivalent of the ADPF NPs by means of tail vein injection. Blood samples were collected from the submandibular vein 0.15, 0.5, 1, 2, 4, 24, and 48 h after administration and processed for pharmacokinetic analyses. The specific operation has been described by Shan et al [45]. A two-compartment pharmacokinetic model was utilized to fit the drug–time curve. The analyses and determination of drug distribution in tissues were similar to the above-mentioned method, but the animal model was A549 tumor-bearing mice. The amount of DOX in the tumor tissues and other major organs were measured by HPLC after 24 h of treatment.
In vivo antitumor activity
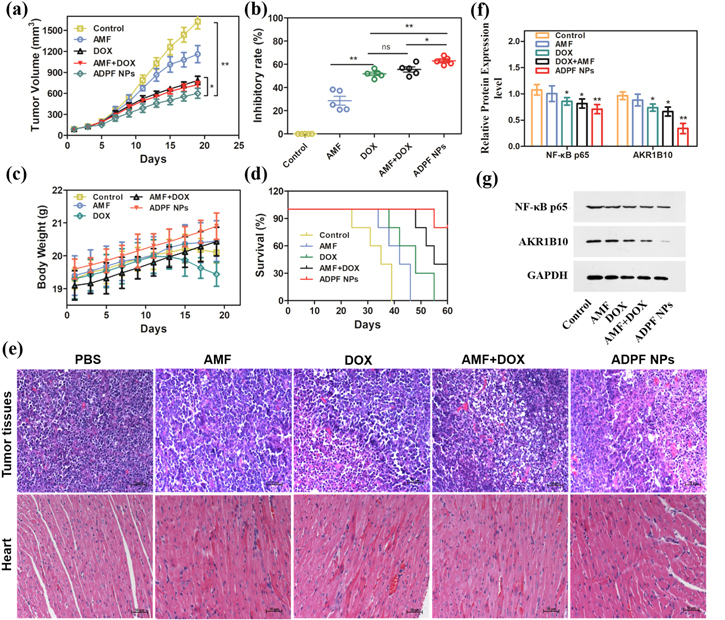
Mice bearing A549 tumors were adopted to evaluate the antitumor activity of the ADPF NPs. The mice were randomly divided into five groups (n = 5) when their tumors grew to about 50 mm3; each group was injected by tail intravenous protocol as follows: (A) control group (PBS buffer), (B) free DOX (2 mg kg−1), (C) free AMF (3.0 mg kg−1), (D) free AMF + free DOX (3.0 mg kg−1, 2 mg kg−1), and (D) ADPF NPs (2 mg kg−1 DOX equivalent). The mice were injected once every other day for three times. The tumor volume and body weight of the mice were measured every other day to evaluate the therapeutic efficacy of each group. The tumor tissues and heart of the mice when killed were collected for pathology and protein level analysis, including AKR1B10 and NF-κB p65.
Statistical analysis
The student's t-test was used to evaluate the statistical significance, which was assigned at *P < 0.05 and **P < 0.01, between groups. Flow cytometry analysis was used with FlowJo Software (TreeStar, Ashland, OR). Additionally, GraphPad Prism v5.0 and Image J software were utilized for data analysis.
Results
Preparation and characterization of ADPF NPs
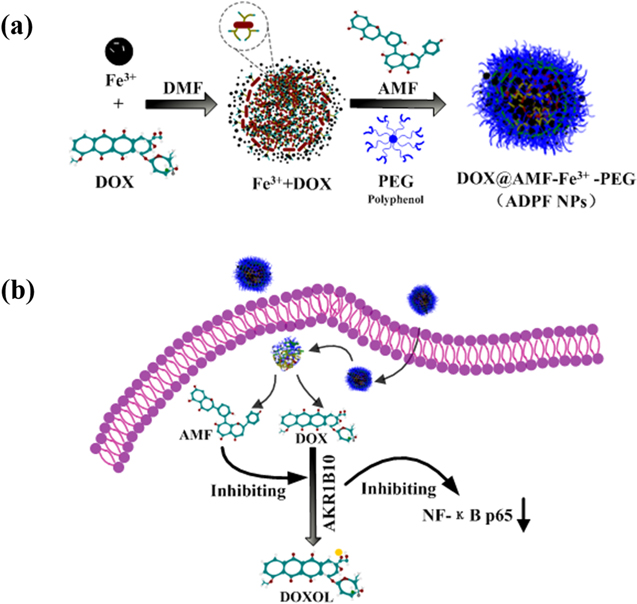
The core of the NPs was formed by the cooperation of hydrophobic DOX with Fe3+ in DMF; then, it was applied for AMF and PEG polyphenol to further assemble AMF@DOX-Fe3+-PEG nanoparticles (ADPF NPs) via complexation with Fe3+ (figure 1(a)). The DOX and AMF wrapped in the ADPF NPs could enhance the synergistic therapy by inhibiting ABR1B10 and NF-κB p65 (figure 1(b)).
Figure 1. Preparation of ADPF NPs and the mechanism of synergistic effects. (a) Schematic of ADPF NPs self-assembly process. (b) The mechanism of ADPF NPs in the intracellular environment.
Download figure:
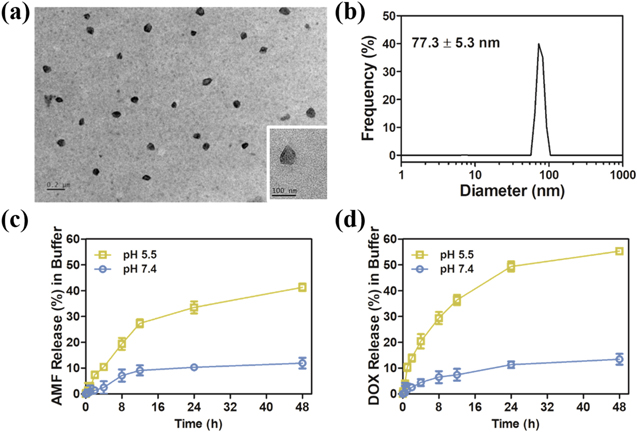
Standard image High-resolution imageAs shown by the TEM images (figure 2(a)), the ADPF NPs presented a round morphology with about 75 nm size, which was consistent with the result of the DLS (figure 2(b)). They exhibited good dispersibility in the aqueous solution, and their zeta potentials were negative charges (−2.92 ± 0.27 mV) (figure S2 (available online at stacks.iop.org/NANO/33/385101/mmedia)), similar to the results of previous reports [43].
Figure 2. Characterization of nanoparticles. (a) TEM images of ADPF NPs. (b) DLS of ADPF NPs in water. AMF (c) and DOX (d) release profile from ADPF NPs under pH 7.4 and 5.5, respectively.
Download figure:
Standard image High-resolution imageAdditionally, HPLC was used to detect the release behaviors of the DOX and AMF encapsulated in the ADPF NPs after they were incubated in PBS at pH 5.5 and 7.4. As shown in figures 2(c) and (d), the release rates of AMF and DOX showed a pH-controlled trait. Overall, their rates at pH 5.5 were much higher than those at pH 7.4. In 24 h, almost 50% of DOX and 34% of AMF were released at pH 5.5. However, at pH 7.4, their rates were both about 10%. After 48 h, the drug release rates of DOX and AMF were 55.29% and 41.31%, respectively, which were more than three times as much as the values at pH 7.4.
The ADPF NPs was stored at 4 °C for eight weeks. The results were shown in table SI. The relevant data, including particle size, PDI, zeta potential, and drug loading of ADPF NPs, did not change considerably. In terms of appearance, the ADPF NPs' water solution was still clear and showed no precipitation.
Cell uptake
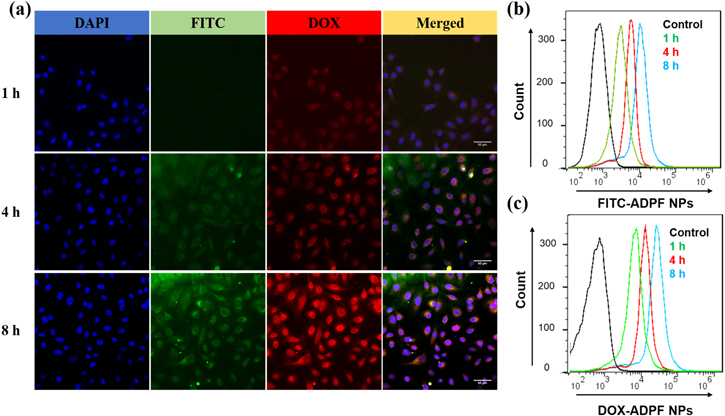
The A549 cells were incubated in Ham's F-12K medium FITC-labeled ADPF NPs containing 6.8 μM of DOX for 1, 4, and 8 h; then, their uptake was determined by confocal microscopy and flow cytometry after the cells were stained with DAPI. The images revealed that the fluorescence intensity of DOX and FITC was enhanced with the incubation time (figure 3(a)). The data from flow cytometry showed that the cell uptake increased sharply from 1 to 8 h (figures 3(b) and (c)). These results suggested that the ADPF NPs were taken in by the A549 cells, and the DOX discharged from the ADPF NPs could accumulate in the cytoplasm.
Figure 3. Cell uptake from A549 cell lines. (a) Confocal microscopy images of A549 cell uptake from FITC-labelled ADPF NPs after incubation for 1, 4, and 8 h. Scale bar: 50 μm. (b)–(c) Flow cytometric analyses of A549 cells uptake by incubating FITC-labelled ADPF NPs.
Download figure:
Standard image High-resolution imageCytotoxicity, cell apoptosis, and Western blot analyses
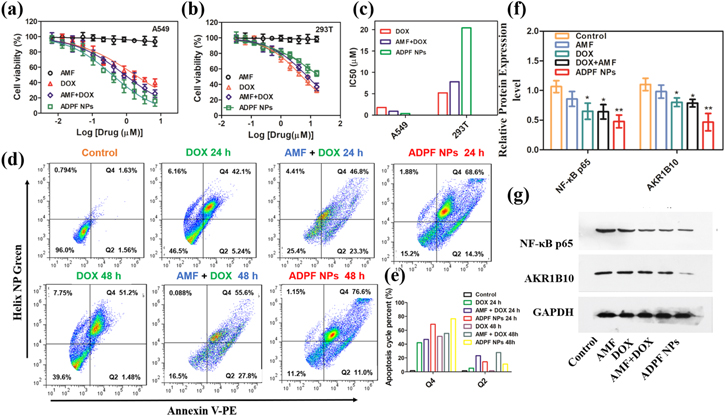
The two cell lines (A549 and HEK 293T) were utilized to investigate the cytotoxicity of the ADPF NPs in vitro via an MTT experiment. As shown in figures 4(a) and (b), the ADPF NPs had dose-dependent toxicity. In the A549 cells, the maximum inhibition rate was close to 90%, followed by the maximum inhibition rates of 74.59% and 60.16% for free DOX + free AMF and free DOX, respectively. The cytotoxicity of free AMF against the two cell lines was negligible. As shown in figure 4(c), the half-maximal inhibitory concentration (IC50) of the ADPF NPs (20.40 μM) was about 2–3 times higher than that of free DOX + free AMF (7.82 μM) and free DOX (5.20 μM), revealing the less cytotoxic effect of ADPF NPs on the 293 cells. On the contrary, the IC50 value of the ADPF NPs was 0.38 μM, which was about 3–5 times lower than that of free DOX + free AMF (0.90 μM) and free DOX (1.77 μM), demonstrating that the ADPF NPs exerted an effective potential antitumor effect against A549 cells.
Figure 4. Cell viability and apoptosis and Western blot analyses after treatment with ADPF NPs. (a) Cell viability of HEK 293T and (b) A549 cells after incubation with ADPF NPs for 48 h. (c) IC50 values of DOX, AMF + DOX and ADPF NPs for A549 cells and HEK 293T cells. (d) Cell apoptosis of A549 cells after treatment with DOX, AMF + DOX and ADPF NPs for 24 and 48 h. (e) Apoptosis cycle percent in Q2 and Q4 zones. (f)–(g) Western blot analyses of AKR1B10 and NF-κB p65 expression from A549 cells after various treatments.
Download figure:
Standard image High-resolution imageThe cell apoptosis of the A549 line was measured using the Dead Cell Apoptosis Kit of Annexin V PE and Helix NPTM Green; then, the percentage of apoptotic cells from early apoptosis (Q2) to late apoptosis (Q4) was determined through quantitative flow cytometry cell apoptosis. The percentages of early and late apoptotic cells were 14.3% and 68.6%, respectively, after incubation in the ADPF NPs for 24 h; these values became 11.0% and 76.6%, respectively, after 48 h (figures 4(d) and (e)). Furthermore, confocal microscopy was used to observe the apoptosis morphology, and the images showed that the apoptosis increased rapidly (figure S5).
Western blot analyses were performed on the A549 cells. As indicated in figures 4(f) and (g), the expression levels of AKR1B10 and NF-κB p65 were significantly suppressed by the ADPF NPs compared with free DOX and free DOX + free AMF. This result reveals that the enhanced apoptosis of A549 by the ADPF NPs was due to the inhibition of the AKR1B10 and NF-κB p65 protein expression.
In vivo acute toxicity and pharmacokinetics
Blood was obtained from the mice's eye socket at 1, 3, and 7 d after injection and was employed for acute toxicity determination. In comparison with the control group, the levels of biochemical parameters (ALT, AST, CK, and CK-MB) in the serum of mice from the ADPF NPs group were not different; however, the four serum parameters of the free DOX group were significantly increased (figures 5(a)–(d)). The H&E stained images of the heart section of mice treated by free DOX and ADPF NPs seven days post-injection indicated that no obvious morphological changes occurred in the heart of the mice in the ADPF NPs group; however, the free DOX group displayed a trifle damage (figure 5(e)).
Figure 5. Blood biochemistry tests on (a) ALT, (b) AST, (c) CK and (d) CK-MB after various treatments for 1, 3 and 7 d (n = 4/group). (e) H&E stained images of heart sections after various administrations for 7 d. Scale bar: 50 μm. (f) Mean drug-time curve of DOX in the plasma of DOX and ADPF NPs (n = 4/group). (g) Amount of DOX present in the tumor tissues and other major organs after treatment (n = 4/group). Notes: *P < 0.05; **P < 0.01.
Download figure:
Standard image High-resolution imageQuantitative determination of plasma DOX levels was used to estimate the pharmacokinetics of free DOX and the ADPF NPs at different times after intravenous injection (figure 5(f)). The plasma half-life of the ADPF NPs (18.95 h) was much longer than that of free DOX (1.08 h), which could increase the accumulation of ADPF NPs to ensure the presence of ADPF NPs in the tumor tissues. The similar results were shown in figure 5(g), indicating that the amount of DOX in tumor tissues from the ADPF NPs group was higher than that in the other major organs.
Antitumor activity
The A549 tumor-bearing nude mice were used to evaluate the antitumor activity of the ADPF NPs. They were randomly divided into five groups for injection with ADPF NPs, free DOX + free AMF, free DOX, and PBS buffer (the control) into the tail vein. Every other day, the tumor volume and body weight were measured. Except for free AMF, the ADPF NPs, free DOX + free AMF, and free DOX could significantly suppress tumor growth (figures 6(a) and S6). The ADPF NPs showed the highest inhibitory rate (62.98%), followed by DOX + AMF (55.51%) and free DOX (51.69%). The inhibitory rate of free AMF was only 28.56% (figure 6(b)). Additionally, the weight of the DOX group decreased significantly, but no obvious weight loss was observed in the mice of the ADPF NPs group (figure 6(c)). Notably, the ADPF NPs could lengthen the life and improve the survival of the mice (figure 6(d)). The images of the tumors and heart tissues stained by H&E indicated that the ADPF NPs had the largest population of dead cells, intercellular blank spots, and necrosis and could improve the cardiac damage caused by DOX (figure 6(e)). These results confirm that the ADPF NPs possessed antitumor activity and could reduce the toxicity of DOX due to AMF-DOX synergistically inhibiting the expression levels of AKR1B10 and NF-κB p65 (figures 6(f) and (g)).
Figure 6. In vivo antitumor evaluation. (a) A549 tumor growth curves. (b) Tumor growth inhibition rates. (c) Body weight changes. (d) Survival curve. (e) Histological evaluation of tumor tissues and heart with H&E. (f)–(g) Western blot analyses of AKR1B10 and NF-κB p65 expression in the tumor tissues. Notes: (n = 5/group; *P < 0.05; **P < 0.01.)
Download figure:
Standard image High-resolution imageDiscussion
Lung cancer is still a concern around the world due to its rapidly increasing incidence and high mortality rate. For example, in the united states, lung cancer mortality is higher than that of prostate, breast, colorectal, and brain cancers according to 2017 statistics; in 2020, the number of new cases of lung cancer ranked second, and the number of deaths was in the top 10 according to the American Cancer Society [46]. In recent years, nanomedicine platforms have been focused on chemotherapy in tumor treatment [47–51]. In this study, ADPF NPs were self-assembled on a combinational nanomedicine platform that was constructed by the chemical complexation of Fe3+ with natural polyphenols (AMF) and their modifications (PEG polyphenols) for drug delivery. The ADPF NPs had a small size, relatively good stability and dispersion in water, and acceptable drug loading efficiency; hence, they could act as intravenous antineoplastic agents.
At present, the numerous PEGylated therapeutics were approved by the FDA for that the PEG-modified NPs have appealing properties of safety, relatively long half-life blood circulation, enhanced permeability, and retention effect [52, 53]. In this study, high-molecular-weight PEG was modified by polyphenols to form ADPF NPs, which possessed a long blood circulation half-life and could improve drug accumulation in the A549 cells. Moreover, the higher DOX release from the ADPF NPs at pH 5.5 than that at pH 7.4 could enhance the predominant antitumor activity targeted at the nucleus of A549 cells. Similarly, the IC50 of the ADPF NPs was significantly lower than that of free DOX, demonstrating the enhanced antitumor efficacy by means of NPs. Conversely, in the HEK 293T cells, the IC50 of the ADPF NPs was higher than that of free DOX and free DOX + AMF, indicating that the cytotoxicity of free DOX to normal cells could be reduced via NPs. These results were further verified by the acute toxicity experiment in vivo.
Recent research has shown that AMF as a natural polyphenol, that is rich in the human diet, can boost the antitumor efficacy of DOX [20]. In this study, the synergistic antitumor effects of ADPF NPs were further confirmed by employing A549 tumor-bearing mice. Compared with the mice treated with free DOX, tumor growth was suppressed by the ADPF NPs and AMF + DOX. Additionally, the largest amount of apoptosis in tumor tissues was observed in the mice of ADPF NPs, followed by AMF + DOX. Meanwhile, the high survival rate and absence of obvious weight loss could mean that the ADPF NPs could reduce the side effect toxicity of free DOX; and reduced toxicity was also achieved, as indicated by the images of the heart tissues stained by H&E. Furthermore, the reduced expressions of AKR1B10 could ameliorate the cardiotoxicity caused by DOXOL produced by DOX. NF-κB p65 as a key protein of the NF-κB signaling pathway involved in the proliferation and migration of tumor cells can be activated by AKR1B10 [16]. Similarly, its expression levels were also inhibited according to the Western blot analyses in vitro and in vivo. It could be responsible for the enhanced synergistic effects of ADPF NPs on tumor apoptosis.
Conclusions
An effective nanoplatform was developed for self-assembling ADPF NPs via the coordination of Fe3+, AMF, and PEG-polyphenol. The ADPF NPs possessed high drug loading efficiency, good stability and dispersion in water, prolonged blood circulation, and pH-dependent release, leading to targeted drug transport and enhanced drug accumulation in the tumor. Moreover, the ADPF NPs could inhibit A549 tumor growth and ameliorate the free DOX-induced cardiotoxicity by reducing AKR1B10 activity. This nanoplatform integrated with AMF and DOX has an extensive application prospect in clinical tumor therapy.
Acknowledgments
This work was supported by Scientific projects research platform of Suzhou University (2020ykf19, 2020ykf24, 2021xhx051), Key research projects of Anhui Provincial Education Department (KJ2021A1109), Provincial natural science foundation of Anhui (1808085MH256, 1908085MC100), International Cooperation projects of Anhui Province (202104b11020017), Suzhou Science-Technology Plan Projects (2019071), Anhui Province Engineering project political course (2020szsfkc1001), Scientific research platform of Suzhou University (2021XJPT33, 2021XJPT36ZC, 2021XJPT37ZC), Provincial college students innovation and entrepreneurship training program (S202110379108), Natural Science Foundation of Suzhou University (2021BSK048).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Competing financial interests
The authors declare no competing financial interest.