Abstract
Graphene and its derivatives have shown fascinating potential in biomedical applications. However, the biocompatibility of graphene with vascular smooth muscle cells (VSMCs) and applications to vascular engineering have not been explored extensively. Using a rat aortic smooth muscle cell line, A7r5, as a VSMC model, we have explored the effects of graphene oxide (GO) on the growth and behaviours of VSMCs. Results demonstrated that GO had no obvious toxicity to VSMCs. Cells cultured on GO retained the expression of smooth muscle cell-specific markers CNN1, ACTA2 and SMTN, on both mRNA and protein levels. A wound healing assay demonstrated no effect of GO on cell migration. We also found that small-flaked GO favoured the proliferation of VSMCs, suggesting a potential of using surface chemistry or physical properties of GO to influence cell growth behaviour. These results provide insight into the suitability of GO as a scaffold for vascular tissue engineering.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Background
Since its isolation in 2004 [1], graphene has attracted tremendous attention due to its unique physicochemical properties. The structure of graphene resembles a hexagonal honeycomb lattice made of a single layer of carbon atoms, which constitutes the basis for its unique properties such as high strength, electrical conductivity, elasticity, flexibility and transparency [2–4], Graphene holds great promise in electronics, energy, structural and biomedical applications [5–8]. Graphene oxide (GO) is one of the most important graphene derivatives. GO is enriched with carboxyl (–COOH), epoxide (–C–O–C–) and hydroxyl (–OH) groups which makes the material to be easily functionalised by biomolecules for tissue engineering and other biomedical applications. As a result, more and more studies have explored its biocompatibility with various types of cells. The use of GO as a scaffold for cell culture and tissue engineering has drawn increasing attention over years. While the toxicological prolife of GO has yet to be fully elucidated, there is increasing evidence indicating little to no toxicity of GO in a range of cells such as mice fibroblasts (L929) [9], NIH-3T3 fibroblasts [10], human fibroblasts and erythrocytes [11], HeLa cells [12], human hepatoma HpG2 cells [13], human embryonic kidney 293 cells [14] and A549 cells [15]. On the other hand, recent reports have also indicated that GO may exert cytotoxicity on human lung fibroblasts [16], neuroblastoma SH-SY5Y cells [17] and human lung epithelial cells (BEAS-2B) [18] in a concentration-dependent manner, possibly as a result of oxidative stress. GO has also been demonstrated as an effective vector for siRNA delivery [19], cancer chemotherapy drug delivery [20], etc and as a non-invasive optical sensor for cancer microenvironments [21]. In light of these discoveries, research in this area is still in its early phases with many aspects of graphene cytotoxicity, biocompatibility and other cellular effects yet to be confirmed. Further exploring the interactions between graphene and different cell types could provide a foundation for safer and better use of GO as platforms for tissue engineering and regenerative medicine, in addition to expanding our current knowledge in this field.
The potential for GO-based composites as scaffolds [22] and drug carriers [23] for vascular tissue engineering has also been explored, however, it has also been claimed that a GO-based composite can inhibit the growth of vascular smooth muscle cells (VSMCs) [24]. Therefore, an understanding of the potential biocompatibility of clean GO towards VSMCs is essential, but to the best of our knowledge, the effects of GO on VSMCs is largely still unknown. Given the fact that VSMCs are the major cell type of blood vessels, and behavioural change of VSMCs involves in a variety of cardiovascular diseases [25], it would be interest to know if GO could affectthe growth and behaviour of VSMCs. Additionally, physical or chemical properties of GO could potentially influence cell phenotypes. We have therefore correlated the growth and behaviours of VSMCs to the flake size of GO which correlates to different density of carboxyl groups and has different physical properties.
In this study, using the rat aortic smooth muscle cell line (A7r5) as a model for VSMCs, we assessed the impact of GO on the cytotoxicity, viability, proliferation, gene expression and migration of VSMCs. We found that GO is highly biocompatible with VSMCs; the VSMCs retained expression of smooth muscle cell-specific markers when cultured on GO surfaces; GO had no effect on cell migration in comparison to glass surfaces. Additionally, A7r5 cells favoured the GO surfaces made by relatively smaller GO flakes for survival.
Methods
Fabrication of GO
GO was prepared through a modified Hummers' method as reported by Rourke et al. [26] 4.5 g KNO3 (Sigma Aldrich, UK) was dissolved in 169 ml H2SO4 (Sigma Aldrich, UK), and 5 g of natural flake graphite (Graphexel, 2369) was added to it. The mixture was cooled down and kept cold by means of an ice bath. 22.5 g KMnO4 (Aesar, UK) was added over 70 min after which time the ice bath was removed and the mixture was left to stir for 3 d, followed by 4 more days left without stirring (the mixture became so thick after 3 d that stirring was no longer possible). Next, 550 ml of 5 wt% H2SO4 in water was added over approximately 1 h and left stirring for another 3 h. 15 g of H2O2 (30% vol.) (Sigma Aldrich, UK) was added drop by drop and subsequently stirred for 2 h. 550 ml of an aqueous solution containing 3 wt% H2SO4/0.5 wt% H2O2 was added and the mixture was left to stir overnight. This mixture was then centrifuged at 8,000 rpm for 20 min and the supernatant was discarded. The pellet, a thick dark yellow liquid, was then dispersed with 500 ml of an aqueous solution containing 3 wt% H2SO4/0.5 wt% H2O2 and shaken in order to fully disperse the pellets. This last step was repeated twelve times until a characteristic glittery colour was not visible. After that, the mixture was washed five times with deionised (DI) water; 500 ml of DI water was added in each washing cycle. GO was further dried under vacuum at room temperature. An aqueous solution of GO at a concentration of 2 mg ml−1 was prepared via sonication for the coating of the coverslips. In order to obtain GO of different particle sizes, the GO stock dispersion of 2 mg ml−1 was allowed to sediment under gravity for 90 d. Large flake GO was obtained by sampling the bottom of the sedimented dispersion while small flake GO was obtained by sampling the supernatant followed by ultrasonication in a low-power bath sonicator for 30 min.
GO characterisation
GO was characterised by atomic force microscopy (AFM), X-ray photoelectron spectroscopy, Raman spectroscopy and UV–vis absorption spectroscopy, as described in the electronic supporting information which is available online at stacks.iop.org/NANO/32/055101/mmedia.
GO coating on glass coverslips
The glass coverslips were washed and plasma treated prior to be coated with GO. The washing was carried out in a sonication bath, immersing the coverslips first in acetone for 15 min, followed by DI water for another 15 min followed by isopropanol for 15 min. The coverslips were then allowed to dry at room temperature. In order to increase the hydrophilicity of the coverslips they were treated in oxygen plasma for 10 min prior to the coating. GO dispersions at a concentration of 2 mg ml−1 were then spin-coated on the glass coverslips at 1,000 rpm for 60 s.
Cell culture
The A7r5 cell line was purchased from American Type Culture Collection (ATCC, Cat No.CRL-1444) and cultured in a monolayer using Dulbecco's Modified Eagle Medium supplemented with 10% Foetal Bovine Serum and 0.5% Penicillin-Streptomycin. Cells were grown in T75 flasks for 5 d prior to passaging on to glass (control) or GO-coated coverslips in 6-well plates. Cells were plated at a density of 2 × 104 cells cm−2 for each well and incubated at 37 °C in a humidified atmosphere with 5% CO2 and 95% air.
Cytotoxicity and cell viability assay
Cytotoxicity and cell viability were detected using the CytoTox-Glo™ Cytotoxicity Assay kit (Promega, Cat.No.G9290) that measures the activity of a distinct protease that is released from the dead cells. Briefly, same numbers of A7r5 cells were cultured on the GO-coated or control glass coverslips, respectively, for 3 d. The cells were trypsinised, and both cells and culture medium were collected and transferred into an opaque 96-well plate. CytoTox-Glo™ Cytotoxicity Assay Reagent was added to the cells according to protocols by the manufacturer. The samples were mixed and incubated at room temperature for 15 min before luminescence measurement was carried out using a GloMax®-Multi + Detection System with Instinct™ Software (Promega). The Lysis Reagent was then added to the reaction, mixed and incubated at room temperature for 15 min. The samples were measured again as the total luminescence signal. Cell viability was calculated as the difference of total luminescent signal (after cell lysis) and the cytotoxicity luminescence signal.
Cell proliferation assay
The proliferation of cells grown on glass or GO surfaces was analysed using CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Cat.No.G7571) that determines the number of viable cells in culture based on quantitation of the ATP present, an indicator of metabolically active cells. Prior to the assay, same numbers of A7r5 cells were grown on GO-coated or control glass coverslips for 3 d. An equal volume of CellTiter-Glo® Reagent to the cell culture medium was added to the cell culture. After mixing for 2 min and incubating at room temperature for 10 min, 200 μl of the reaction was transferred into opaque 96-well plate, and luminescent signal was obtained using a plate reader and recorded by a GloMax®-Multi + Detection System with Instinct™ Software (Promega).
A second assessment of cell proliferation was performed by immunofluorescent staining for antigen Ki-67 (as described in the Immunocytochemistry section). Three random sections on each coverslip were chosen and photographed at ×20 magnification using Leica Application Suite software. The number of Ki-67-positive cells and the total number of cells were then counted using ImageJ software.
Quantitative reverse transcriptase PCR (RT-qPCR)
A7r5 cells were grown for 3 d on the glass control or GO surfaces. Total RNA was extracted using the RNeasy® Mini Kit (Qiagen, Cat.No.74104). The cDNA was synthesised from 1.0 μg total RNA using Tero cDNA Synthesis Kit (Bioline, Bio-65042) in a 20 μl reaction. Primer sequences were listed in table 1. The cDNA samples were diluted to 10 ng μl−1, and the following mix was prepared in 96-well non-skirted optical PCR plates (Cat.No.E1403-0200, Starlab) for qPCR reaction: 10 μl Power SYBR Green PCR Master Mix (Life Technologies), 0.5 μl forward primer, 0.5 μl reverse primer, 2 μl cDNA and 7 μl dH2O. QPCR was performed on a StepOnePlus™ Real-Time PCR System (Applied Biosystems®). The primers used for qPCR were purchased from Eurofins (Luxembourg) and were specific for rat calponin (CNN1), α-smooth muscle actin (ACTA2) smoothelin (SMTN), actin β (ACTB) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Relative expression of mRNA was normalised to the expression of housekeeping genes (ACTB or GAPDH).
Table 1. Primers sequences used in RT-qPCR.
| Forward primer | Reverse primer | |
|---|---|---|
| ACTB | 5' CCA CCA TGT ACC CAG GCA TT 3' | 5' ACG CAG CTC AGT AAC AGT CC 3' |
| GAPDH | 5' ATT GTT GCC ATC AAC GAC CC 3' | 5' GGT TCA CAC CCA TCA CAA AC 3' |
| CNN1 | 5' CGG CGT CAC CTA TAT GAT CC 3' | 5' AGC GTG TCA CAG TGT TCC AT 3' |
| ACTA2 | 5' CAT CAG GAA CCT GCA GAA GCT G 3' | 5' CCA TTC CAA CCA TCA CTC CCT 3' |
| SMTN | 5' CAT CAG GAA CCT GCA GAA GCT G 3' | 5' CCC GAA GAG CCT TCC TTC TC 3' |
Immunocytochemistry
A7r5 cells grown on glass or GO-coated coverslips were fixed in 4% paraformaldehyde for 20 min, permeabilised in 0.1% Triton X-100 for 10 min and blocked with 5% fetal bovine serum in phosphate buffered saline (PBS). Cells were then incubated with primary antibodies against calponin 1 (ab46794, Abcam), α-smooth muscle actin (α-SMA) (1A4, DAKO) and antigen Ki-67 (ab16667, Abcam) for 1 h at room temperature. After washing with PBS, the fluorescent secondary antibodies (Alexa Fluor 488 or 594, Life Technologies) were added and incubated for 1 h. The cell nuclei were counterstained with DAPI (Sigma). Cells were visualised under a Leica inverted microscope using DAPI, TRITC, FITC filters (Leica).
Cell migration assay
A 10 μl pipette tip was used to create three scratch line gaps in the monolayer of confluent cells cultured on glass or GO-coated coverslips, respectively. Each line gap was visualised and photographed every 2 h over a total period of 8 h using a Leica inverted microscope and Leica Application Suite software, respectively. ImageJ software was used to measure the widths of the line gaps at each time point (i.e. 0, 2, 4, 6 and 8 h).
Statistical analysis
Statistical data were expressed as mean ± SEM and were analysed by a two-tailed t-test. Statistical significance was determined when p < 0.05. * represents p < 0.05 and ** represents p < 0.01.
Results
GO exhibits no cytotoxic effect on VSMCs
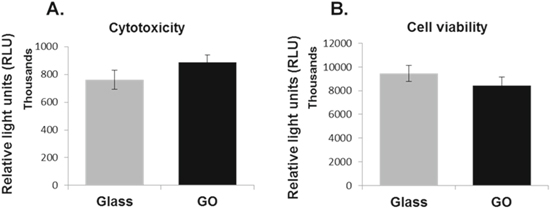
A7r5 cells cultured on the GO-coated coverslips and the glass control surfaces for 3 d were subject to cytotoxicity assay. Although the luminescence reading for cells growing on GO surfaces appeared slightly higher than that of the control, the difference was not statistically significant (p = 0.1618), suggesting a low toxicity of GO to VSMCs (figure 1(A)).
Figure 1. Influences of graphene oxide on cytotoxicity and viability of VSMCs. Vascular smooth muscle cell line A7r5 were cultured on glass coverslips with or without GO coating for 3 d. Cytotoxicity (A) or cell viability (B) were determined using the Promega CytoTox-Glo™ Cytotoxicity Assay kit. Data are mean ± SEM, n = 3. GO, graphene oxide.
Download figure:
Standard image High-resolution imageEffects of GO on cell survival and proliferation
The cytotoxicity luminescent assay kit was also used to measure the influence of GO on the survival of A7r5 cells by lysing all the cells after toxicity assay to obtain the total luminescence and presenting the data as the difference between the total and toxicity luminescent signals. As shown in figure 1(B), there is no statistical difference on the viability luminescent signals between cells growing on GO and the glass control surfaces (p = 0.3051), suggesting the GO surface had no significant impact on cell survival or proliferation.
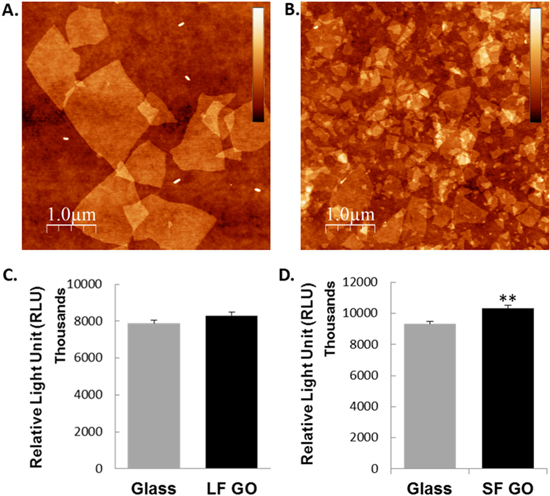
GO exists as flakes in micron or submicron scales. It is possible that physical or chemical effects associated with the edges of the GO could potentially modulate cell behaviours [27]. We therefore produced smaller GO flakes [(0.4 ± 0.1) × (0.3 ± 0.1) μm], named SF, than the original relatively larger GO flakes (LF) as used above [(2.4 ± 0.8) × (1.6 ± 0.5) μm] (figures 2(A), (B)). Cell viability or proliferation were again evaluated for A7r5 cells growing on the surface of the LF GO using a different assay kit that was based on quantitation of cellular ATP, an indicator of metabolically active cells. In line with data obtained above, no statistical difference was observed between cells growing on the LF GO-coated and the control glass coverslips (p = 0.0875, figure 2(C)). However, cells grown on the SF GO-coated coverslips had a significantly higher proliferation rate (p = 0.006) than cells growing on the glass control surfaces (figure 2(D)).
Figure 2. Role of graphene oxide on the proliferation of VSMCs. GO was produced as either larger flakes (LF GO, (A)) or smaller flakes (SF GO, (B)). AFM images are 10 × 10 μm. A7r5 cells were grown on glass coverslips with or without LF GO or SF GO coating for 3 d. The proliferation or survival of the cells was determined using the Promega CellTiter-Glo® Luminescent Cell Viability Assay kit (C) and (D). Data are mean ± SEM, n = 3. SF GO, small-flake graphene oxide; LF GO, large-flake graphene oxide.
Download figure:
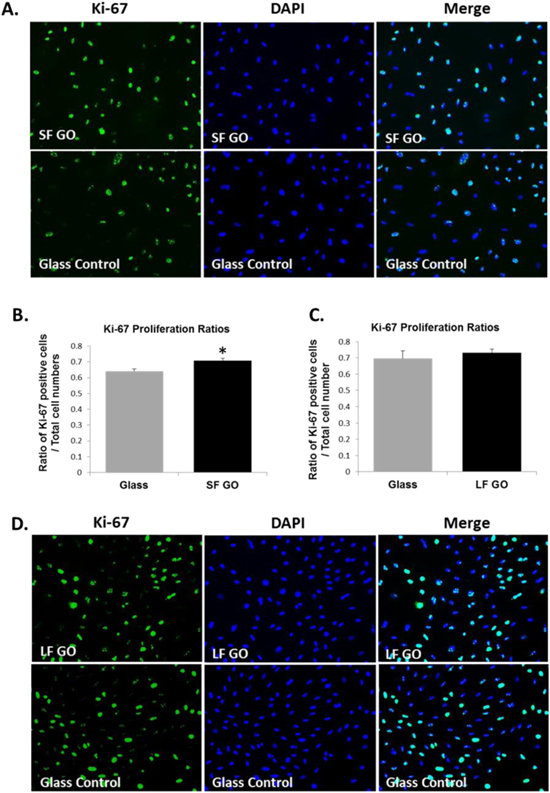
Standard image High-resolution imageIn line with these data, immunofluorescent staining of Ki-67, a known proliferation marker, showed that an increased proportion of Ki-67 positive A7R5 cells were observed when the cells were cultured on the SF GO-coated surfaces compared to the glass surface control (figures 3(A), (B)), while the proportion of Ki-67-positive cells were no significant difference between the LF GO-coated coverslips and glass surfaces (p = 0.5350; figures 3(C), (D)). The results have confirmed that smaller GO flakes promoted growth and proliferation of VSMCs.
Figure 3. Immunofluorescent staining of proliferation marker Ki-67 for VSMCs grown on GO surfaces. A7r5 cells were grown on glass coverslips with or without GO coating for 3 d. Immunofluorescent staining for ki-67 (green) was carried out on the cells that were grown on SF GO (A) or LF GO (D), respectively, and counter stained for nuclei using DAPI (blue). Ratios of the number of Ki-67 positive cells to total cell number were counted for each substrate on 3 random views and presented as bar graph ((B) for SF GO, and (C) for LF GO). Data are mean ± SEM, n = 3. SF GO, small-flake graphene oxide; LF GO, large-flake graphene oxide.
Download figure:
Standard image High-resolution imageA7r5 cells retain expression of VSMC specific markers when cultured on GO
To verify the influence of GO on the phenotype of VSMCs, A7r5 cells were grown on the LF GO, SF GO or glass surfaces, respectively, and VSMC specific contractile markers, CNN1, ACTA2, and SMTN were quantified by RT-qPCR. Results showed that, regardless of substrate surfaces, no significant differences were found in mRNA levels of any of the gene tested when comparing cells growing on the GO or glass control surfaces, suggesting that GO is unlikely to change the contractile phenotype of VSMCs, regardless the GO flake size (figures 4(A), (B)).
Figure 4. RT-qPCR results showing expressions marker genes of VSMCs when growing on GO surfaces. A7r5 cells were grown for 3 d on the glass control or GO coated coverslips. The mRNA expression of VSMC specific marker genes, CNN1, ACTA2 and SMTN, were determined using RT-qPCR. (A) Effect of LF GO on VSMC gene expression; (B) effect of LF GO on VSMC gene expression. Data are mean ± SEM, n = 3. SF GO, small-flake graphene oxide; LF GO, large-flake graphene oxide.
Download figure:
Standard image High-resolution imageImmunofluorescent staining for calponin 1 and α-SMA was also performed to verify the VSMC phenotype. As it can be observed in figure 5, A7r5 cells cultured on glass, LF GO-coated or SF GO-coated coverslips were all stained positively for the two markers, which indicated that the cells retained the expression of smooth muscle cell-specific markers at protein level.
Figure 5. Immunofluorescent staining of specific marker proteins for VSMCs that grown on surfaces of graphene oxide or controls. A7r5 cells were grown for 3 d on the glass control (a)–(e), LF GO (f)–(j) or SF GO (k)–(o) coated coverslips followed by immunofluorescent staining of VSMC specific markers, Calponin 1 (red) or α-SMA (green). The cells were counter stained by DAPI (blue). SF GO, small-flake graphene oxide; LF GO, large-flake graphene oxide.
Download figure:
Standard image High-resolution imageGO does not affect A7r5 cell migration
Migration is an important measure of VSMC function. An increased migration of VSMCs correlates to a dedifferentiated phenotype and contributes to a number of vascular conditions including restenosis after angioplasty and atherosclerosis. To determine if GO changes the migration properties of VSMCs, we adapted a wound healing process to monitor the migration of VSMCs. A wound scratch was made on the monolayer of A7r5 cells that were grown on the surface of LF GO, SF GO or the glass, and the ability of the cells to migrate and repair the wound scratch were measured. As observed in figure 6, cells migrated at similar rates to close up the wound gaps, irrespective of the substrate material (glass versus GO) or the size of the GO flakes, suggesting GO has no significant effect on the migration of VSMCs.
Figure 6. Determination of the migration of VSMCs grown on graphene oxide surfaces. A7r5 cells were grown on the glass control (a)–(e) or GO coated (f)–(j) coverslips until confluent. Wound scratches were created using a pipette tip. The distance of cell migration to heal the wound gaps were measured. Light microscopy of A7r5 cells grown on LF GO (A) or SF GO (B) surfaces. Quantifications of the migration of A7r5 cells grown on surfaces of LF GO (C) or SF GO (D). Data are mean ± SEM, n = 3. SF GO, small-flake graphene oxide; LF GO, large-flake graphene oxide.
Download figure:
Standard image High-resolution imageDiscussion
GO has by now been considered to be a promising material in biomedical applications. GO can be coated on medical implant surfaces or incorporated into scaffolds for either functionalizing the biomaterial or acting as a bio-sensor [28]. With growing applications of GO in areas of tissue engineering, it is urgently needed to evaluate its potential risks and have a fundamental understanding of the interaction between GO and surrounding cells. In this study, we examined the effects of GO-coated surfaces on the growth behaviours of VSMCs, in an attempt to establish the biocompatibility of GO with vascular cells and advance our knowledge on its suitability as a scaffold for vascular tissue engineering.
VSMCs are an integral part of blood vessels and play key roles in maintaining the integrity of the vessel structure, regulating blood pressure, and contributing to tissue repair following injury or insult. To perform such diverse functions, VSMCs benefit from the ability of switching between different phenotypes. Throughout embryonic development and blood vessel repair process, VSMCs exhibit a partly differentiated phenotype also termed 'synthetic' phenotype. During this time, VSMCs are characterised by extensive proliferative and migratory properties as well as secretion of extracellular matrix proteins. Following maturation of the circulatory system or complete repair of an injured blood vessel, VSMCs differentiate to a mature 'contractile' phenotype [29]. However, VSMCs tend to lose the contractile property when they are dedifferentiated in response to vascular injury. Such phenotype change makes VSMCs become migratory, which contributes to the neointimal formation and vascular remodeling. This property should be avoided when considering materials for vascular scaffold in vascular engineering. Using a rat aortic SMC line, the A7r5 cells, our results revealed that GO did not cause phenotype switch of VSMCs as evidenced by the lack of changes in the expressions of VSMCs specific marker genes. The lack of influence of GO on VSMCs migration provided additional evidence for the suitability of GO to be used for vascular grafts or implants.
GO is a two-dimensional material composed of a sp2 bonded carbon network. It is a highly oxidized form of graphene containing carboxyl group (–COOH) in the edges and hydroxyl (–OH) and epoxy (–C–O–C–) group in the basal planes [30, 31]. The properties of GO as soft membranes with high in-plane stiffness and high surface energy through bonded oxygen groups have many advantages for various applications, including the biomedical field. The functional groups present on the graphene surface enable increased interaction with proteins through electrostatic, covalent, and hydrogen bonding. Hence, the increased proteins in the GO membrane strongly improve cell adhesion of cells attaching on the GO surface [32–34], which could potentially modulate cell survival. Theoretically, GO surface made of smaller GO flakes would have higher density of –COOH group than that of the larger flakes. It is known that the –COOH group could alter cell growth behaviours [35] and significantly enhance cell proliferation [36], we employed GO with two different flake sizes and found that the VSMCs cultured on small-flake GO displayed higher levels of survival and proliferation when compared to the larger flake GO surfaces. However, smaller GO flake covered surfaces also have higher surface roughness (Rq = 0.740 nm) compared to large GO flake covered surface (Rq = 0.657 nm) and bare glass surface (Rq = 0.220 nm), which could also potentially modulate cell growth and behaviour. Future experiments could explore in details how the growth and behaviours of VSMCs are changed by surface chemistry and physical properties of GO.
In summary, we have shown in this study that GO is a biocompatible material to VSMCs. GO did not exhibit significant cytotoxicity to VSMCs and had no influence on VSMC viability. VSMC-specific markers, CNN1, ACTA2 and SMTN, remained unchanged on both transcriptional and protein levels when the cells were grown on the surface of GO as compared to the glass control surfaces. Additionally, GO did not change the migration of VSMCs. Furthermore, we found that VSMCs grown on smaller GO flakes surface, which contained higher carboxyl group density as well as higher surface roughness, tended to have an increased proliferation rate compared to cells grown on glass surfaces. These results contribute to our current understanding of GO on vascular cells growth and behaviours, and provide insight into the suitability of GO as a scaffold for its future applications in vascular tissue engineering or implants.
Acknowledgments
We thank the MRes programme on Translational Medicine in the University of Manchester which supported GB's project. AV and PG acknowledge funding from the Engineering and Physical Sciences Research Council (grant EP/K016946/1).