Abstract
The optical properties of CsPbCl3 perovskite nanocrystals (NCs) with varied sizes were studied in the temperature range from 80 to 320 K by steady-state and time-resolved photoluminescence (PL) spectroscopy. The CsPbCl3 NCs were synthesized with a hot-injection approach at reaction temperature of 140–180 °C. The PL emissions in NC films originate from localized excitons. It is found that NC films shows a significant decrease in PL intensity with increasing temperature while they exhibit a clear increase in PL lifetime from 80 K to around 250 K and then a reduction at high temperature. The abnormal temperature dependence of PL lifetimes in NC films is related to thermal activation of trapped carriers in the NCs. The change of average lifetimes with emission energy indicates the thermal degradation result from the loss of ligands on the surface of NC films. Moreover, the PL intensities, peak energies, and bandwidths of the NC films as a function of temperature are discussed detail.
Export citation and abstract BibTeX RIS
1. Introduction
In recent years, inorganic-metal halide perovskite nanocrystals (NCs) have exhibited outstanding features such as easy solution processing, good quantum yield, and highly saturated color. Therefore, they have wide and promising applications in photodetectors, photocatalysis, radiation detection, light emitting diodes (LEDs), solar cells, and fluorescence labeling. Moller first reported the crystal structure of inorganic lead halide perovskite CsPbX3 (X=Cl, Br, I) NCs in 1958, but its fluorescence properties had not yet been studied at the time [1]. By 2009, with the advent of perovskite solar cell research, inorganic lead halide perovskite CsPbX3 (X=Cl, Br, I) based luminescent materials had been developed rapidly [2–5]. Recently, highly luminescent all-inorganic perovskite CsPbX3 (X=Cl, Br, I) NCs were synthesized by hot-injection and/or anion exchange approaches, with emission quantum efficiency of above 90%, full width at half maximum (FWHM) of less than 50 nm and tunable photoluminescence (PL) emission from 400 to 700 nm [6, 7]. More research and improvements on the optical properties of perovskite NCs can help them be successfully applied in the field of solid state lighting and displays.
Up to now, few studies have reported on the thermal stability and luminescence mechanism of perovskite NCs with different sizes. The excitons produced by low-density excitation are almost completely ionized at room temperature, if the exciton binding energy is much larger than the thermal energy at room temperature, which has been demonstrated by D'Innocenzo et al [8]. Besides, He et al revealed that the recombination of photo-generated carriers is dominated by excitons localized for organolead trihalide perovskites in band tail states. The excellent power-law dependence of the excitation power was determined to be 1.179 using experiments of the excitation density-dependent luminescence and spectral-dependent luminescence decay, indicating that the radiative recombination of organolead trihalide perovskites is dominated by excitons [9]. In addition, the exciton-phonon coupling and nonradiative relaxation processes in PbS and CdSe colloidal quantum dots were studied with the temperature-dependent PL spectroscopy [10–12]. However, a systemic study about the luminescence mechanism and spectroscopy properties for all-inorganic perovskite NCs has not yet been provided in previous reports. Therefore, it is necessary to study the recombination and degradation mechanisms of the PL in all-inorganic perovskite films to further understand their luminescent properties and thus improve their thermal stability of luminescence.
In this work, the quenching mechanism and thermal stability of PL emissions in CsPbCl3 NCs with different particle sizes are discussed. The PL intensities, peak energies, linewidths, and lifetimes of the CsPbCl3 NCs as a function of temperature are shown in detail. In addition, the structure and luminescent properties of NCs based on transmission electron microscopy (TEM), x-ray diffraction (XRD), and temperature-dependent PL spectroscopy were characterized.
2. Experimental
2.1. Synthesis of CsPbCl3 NCs
The CsPbCl3 NCs were synthesized with a hot-injection approach. First, octadecene and PbCl2 were loaded into a 50 ml 3-neck flask to obtain a mixed solution, it was then degassed for 30 min at 120 °C. Oleylamine and oleic acid were injected into the 3-neck flask at 120 °C under N2 protection. When the PbCl2 was completely dissolved, the temperature of the reaction mixture was elevated to 110–180 °C and the inject Cs-oleate precursor solution was rapidly obtained by dissolving CsCO3 in oleic acid and octadecene at 150 °C. After 5 s, the heating for the reaction mixture ceased and it was cooled via an ice-water bath. Finally, the CsPbCl3 NCs obtained by centrifugation repeatedly at 5000 rpm were dissolved in hexane.
2.2. Characterization
The absorption spectrum measurements were carried out with a Shimadzu UV-2700 UV-visible spectrophotometer. A transmission electron microscope (TEM, JEOL JEM2100) operated at 200 kV was utilized to characterize the morphologies of the CsPbCl3 NCs. The XRD patterns were measured using a Rigaku D/max-2500 diffractometer with CuKα radiation at room temperature. The PL spectra, temperature-dependent PL spectra, quantum yields and decay curves were performed on a spectrometer (Horiba JobinYvon Fluorolog-3) with a time-correlated single-photon counting system and a quantum yield accessory.
3. Results and discussion
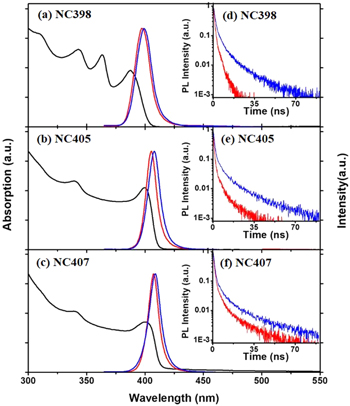
The normalized UV-visible absorption, PL spectra and decay curves of CsPbCl3 NCs are shown in figure 1. As seen in figure 1, like CdSe quantum dots, the exciton absorption bands with well resolved peaks of CsPbCl3 NCs are clearly observed. The exciton absorption peaks of CsPbCl3 NCs shifts to the blue and become sharp with decreasing the cube size. The peak wavelengths (energies) of exciton absorption bands were estimated to be 387 nm (3.20 eV), 399 nm (3.10 eV), and 400 nm (3.09 eV), respectively. As shown in the PL spectra of CsPbCl3 NCs in hexane, the peak wavelength positions of the three NC samples are located at 398, 405 and 407 nm; therefore, they can be labeled as NC398, NC405 and NC407, respectively. The full width at half maximum (FWHM) of CsPbCl3 NCs for NC 398, NC 405 and NC 407 is 15.1 nm (119.2 meV), 10.8 nm (81.7 meV) and 10.7 nm (79.8 meV). Their Stokes shifts are 10.3 nm (82.6 meV), 5.6 nm (42.9 meV) and 6.7 nm (50.9 meV), respectively.
Figure 1. Normalized UV-visible absorption (black lines), PL spectra and PL decay curves (illustrations) of CsPbCl3 NCs with an emission peak at 398, 405 and 407 in hexane. The red and blue lines represent CsPbCl3 solution and CsPbCl3 NC films, respectively.
Download figure:
Standard image High-resolution imageIn figure 1, the CsPbCl3 NC films for NC398, NC405 and NC407 show an emission band at 399, 408, and 409 nm with FWHM of 15.25 nm (118.7 meV), 12.2 nm (90.9 meV) and 12.1 nm (90.3 meV). The emission from NC films shows a red-shift about 14.36, 18.74, and 6.19 meV, respectively, compared with their CsPbCl3 NCs in hexane, which means energy transfer in the CsPbCl3 NCs of different sizes. Figures 1(d)–(f) show the PL decay curves of the CsPbCl3 NCs. Their obtained PL lifetimes were 1.22 ns and 4.86 ns for NC398, 3.07 ns and 6.50 ns for NC405 and 3.87 ns and 7.06 ns for NC407 in hexane (red line) and deposited on quartz substrates (blue line), respectively. It is clearly visible that the NC films exhibit longer PL lifetime than the NCs in solution, which is in contrast to the shortening of PL lifetimes in CdSe quantum dots films due to energy transfer from smaller dots to the larger ones.
The TEM images and XRD patterns of CsPbCl3 NCs are shown in figure 2. Figure 2(a) shows a typical TEM image of the CsPbCl3 NC films for NC 398, and the NCs are cube-like in shape with an average grain size of 6.1 nm. The NCs for NC405 and NC407 have different average grain sizes of 8.3 and 9.8 nm, respectively. Clear lattice fringes of NCs for NC398, NC405 and NC407 in the high-resolution TEM images are seen in illustration, which show the good crystallinity of the synthesized NCs. The cubic phase of CsPbCl3 NC films for NC398, NC405 and NC407 has been further confirmed by the XRD patterns according to (JCPDS card No. 54-0752), as shown in figure 2(d). There are only the (100) and (200) diffraction peaks that were observed, indicating that CsPbCl3 NC films have (100) preferred orientation.
Figure 2. The TEM images for NC398 (a), NC405 (b), and NC407 (c) and the high-resolution TEM images (illustration) of CsPbCl3 NC films. The XRD patterns (d) of CsPbCl3 NC films.
Download figure:
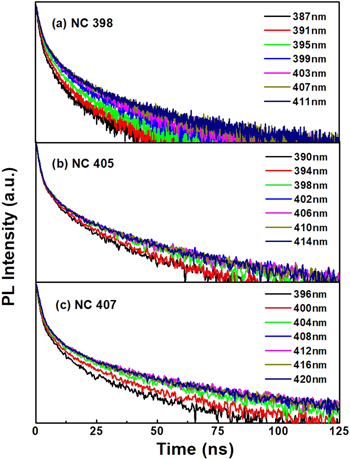
Standard image High-resolution imageThe PL decay curves of CsPbCl3 NC films for NC407 (a), NC405 (b) and NC398 (c) monitored at different emission wavelength at room temperature are shown in figure 3. As can be seen from the graph, the PL lifetimes of the three samples are almost unchanged in the long wavelength side, while the lifetimes decrease with the wavelength decrease. The PL decay curves can be described by the stretched exponential model [13, 14],
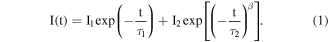
Here, I1 and I2 are the weight factor,  and
and  are the decay time and
are the decay time and  is the stretching parameter about the dimensionality of the localized centers. We found that both
is the stretching parameter about the dimensionality of the localized centers. We found that both  and
and  exhibit obvious spectral dependence. The PL lifetime for excitons localized in the tail states at different emission energies can be fitted by [15]:
exhibit obvious spectral dependence. The PL lifetime for excitons localized in the tail states at different emission energies can be fitted by [15]:
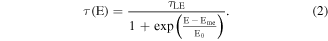
where the first term  is the lifetime of localized excitons,
is the lifetime of localized excitons,  can be regarded as the energy for radiative lifetime,
can be regarded as the energy for radiative lifetime,  is an average localization energy. That is a characteristic energy of the density of band tail states. Using equation (2) obtains the radiative lifetime of
is an average localization energy. That is a characteristic energy of the density of band tail states. Using equation (2) obtains the radiative lifetime of  = 3.12 ns,
= 3.12 ns,  = 3.22 eV and
= 3.22 eV and  = 73.7 meV for NC 407 (a),
= 73.7 meV for NC 407 (a),  = 2.72 ns,
= 2.72 ns,  = 3.21 eV and
= 3.21 eV and  = 46.7 meV for NC 405 (b) and
= 46.7 meV for NC 405 (b) and  = 4.81 ns,
= 4.81 ns,  = 3.28 eV and
= 3.28 eV and  = 86.0 meV for NC398 (c), respectively. It is observed that almost the entire PL of the three samples is emitted at energies under the corresponding
= 86.0 meV for NC398 (c), respectively. It is observed that almost the entire PL of the three samples is emitted at energies under the corresponding  , which indicates that the recombination dynamics in CsPbCl3 NC films is dominated by localized excitons.
, which indicates that the recombination dynamics in CsPbCl3 NC films is dominated by localized excitons.
Figure 3. The PL decay curves of CsPbCl3 NC films for NC407 (a), NC405 (b) and NC398 (c) at various emission wavelengths.
Download figure:
Standard image High-resolution imageFigure 4 shows the PL decay curves of CsPbCl3 NC films for NC407 (a), NC405 (b) and NC398 (c) at the various temperatures from 80 K to 320 K. The lifetime of the three samples showed a trend of increasing initially and then decreasing with the increase of temperature. The decay curves were presented on a spectrometer with a time-correlated single-photon counting spectrometer excited at a wavelength of 302 nm.
Figure 4. The PL decay curves of CsPbCl3 NC films for NC 407 (a), NC 405 (b) and NC 398 (c) samples in the temperature range of 80–320 K.
Download figure:
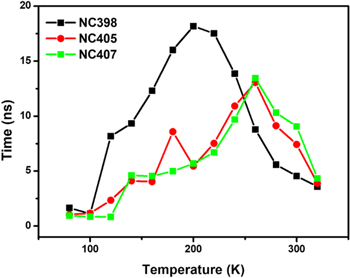
Standard image High-resolution imageThe average PL lifetimes of CsPbCl3 NC films in the temperature range of 80–320 K are shown in figure 5. It is noted that the average PL lifetimes of all the samples become shorter suddenly when the temperature reached 200 K for NC398, and when the temperature increase to 260 K for NC405 and NC407, respectively. The PL lifetimes can be fitted using a binomial exponential function [16]:
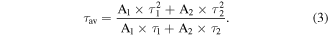
Accordingly, the CsPbCl3 NC film for NC398 has a PL lifetime of 1.65 ns at 80 K, 18.17 ns at 200 K and 3.59 ns at 320 K. The PL lifetimes of NC films for NC405 and NC407 were determined to be 1.06 ns and 0.95 ns at 80 K, 13.05 ns and 13.44 ns at 260 K and 3.89 ns and 4.34 ns at 320 K, respectively. The increases in the average lifetimes with temperature is different than the results in past reports for most colloidal quantum dots attributed to the emission derived from exciton recombination. While, recently, the phenomena that the average lifetimes of colloidal CdSe quantum dots increase with increasing temperature has been observed, which is due to introducing a charge trapping state or a localized state as a relaxation pathway [17, 18]. Besides, as seen in figure 7(a), a rapid decrease in PL intensities of CsPbCl3 NC films with the increase of temperature has been observed, which indicates that plenty of defect states exist in NC films. In other words, the localized states or surface states may exist in the CsPbCl3 NC films, which perhaps explain the lengthening of the PL lifetimes for NC films. In addition, a rapid decrease in the average PL lifetimes of CsPbCl3 NC films with the further increasing temperature has been observed in figure 5, which may be due to the thermal degradation result from the loss of ligands on the surface of NC films.
Figure 5. The average PL lifetimes of CsPbCl3 NC films at various temperatures.
Download figure:
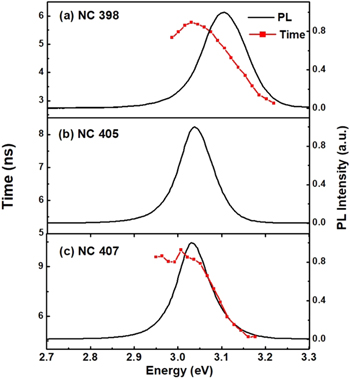
Standard image High-resolution imageIn order to understand the situations of defects at various positions in CsPbCl3 NC films, the average lifetimes were measured at different emission energies. The PL spectra and average lifetimes of CsPbCl3 NC films for NC407 (a), NC405 (b) and NC398 (c) as a function of emission energy at room temperature are shown in figure 6. It can be seen that the average lifetimes of all three samples remain constant at the low-energy side, while observably decreasing with the increase of emission energy. The lifetime of the localized excitons is the longest due to there being few defects, this is consistent with previous results.
Figure 6. The PL spectra and lifetimes of CsPbCl3 NC films for NC407 (a), NC405 (b) and NC398 (c) as a function of emission energy.
Download figure:
Standard image High-resolution imageFigure 7 shows the temperature-dependent PL spectra of CsPbCl3 NC films for NC407 (a), NC405 (b) and NC398 (c) at various temperatures from 80 K to 400 K. The PL intensities of three NC films decreased obviously with the increase of temperature, in addition, the peak energies of these NC films presented large blue shift and the PL emission linewidths exhibited clear increase with increasing temperature. The PL intensities of NC films are normalized at 80 K.
Figure 7. Temperature-dependent PL spectra of CsPbCl3 NC films for NC407 (a), NC405 (b) and NC398 (c) in the temperature range of 80–400 K.
Download figure:
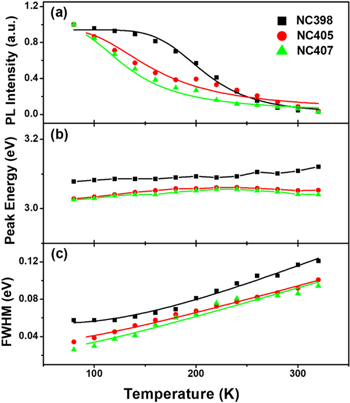
Standard image High-resolution imageThe temperature-dependent PL intensities, peak energies and linewidths of CsPbCl3 NC films for NC 398 (black), NC 405 (red) and NC 407 (green) as a function of temperature are shown in figure 8. The PL intensities of the NC films gradually decrease as the temperature rises from 80 K to 320 K as shown in figure 8(a), which perhaps are due to the increasing nonradiative recombination centers. The integrated PL intensities of the three samples for NC 398 (black), NC 405 (red) and NC 407 (green) can be fitted with the Arrhenius equation [19, 20]:
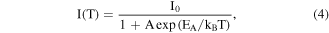
where I0 is the initial intensity at 80 K, A is a constant, kB is the Boltzmann constant and EA is the activation energy. The activation energies of the NC films for NC407 (green), NC405 (red) and NC398 (black) were obtained to be 51.71, 55.68, and 68.12 meV, respectively, which observed that the activation energies decrease with the increasing of particle size. These results of the activation energies for the NC films indicate that the three samples themselves have many nonradiative recombination centers. The peak energies of the CsPbCl3 NC films for NC407 (green), NC405 (red) and NC398 (black) are confirmed to be 3.03, 3.12, and 3.08 eV at 80 K and 3.09, 3.06, and 3.05 eV at 320 K, respectively, as shown in figure 8(b). The three samples exhibited slightly blue shift of PL peak with the temperature increase, which can be attributed to the electron–phonon coupling of quantum dots. The linewidths of PL emissions for the CsPbCl3 NC films for NC407 (green), NC405 (red) and NC398 (black) as a function of temperature were fitted in order to study the exciton-phonon coupling of NC films as shown in figure 8(c). The linewidths for the NC films were obtained with the following expression [21]:
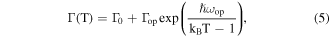
where Γ0 is the inhomogeneous line width which arises from the inhomogeneous of shape, size, and compositions of quantum dots, Γop is the exciton–optical phonon coupling coefficients, kB is the Boltzmann. ħωop is the optical phonon energy which were fitted to be 17.32, 20.28, and 37.87 meV for the CsPbCl3 NC films for NC407 (green), NC405 (red) and NC398 (black), respectively. It can be seen that the optical phonon energies of the three samples are close to the phonon energy obtained using Raman spectroscopy previously [22].
Figure 8. Temperature-dependent PL intensities (a), peak energies (b), and linewidths (c) of CsPbCl3 NC films for NC398 (black line), NC405 (red line) and NC407 (green line) samples in the temperature range of 80–320 K.
Download figure:
Standard image High-resolution image4. Conclusion
In summary, the optical properties of CsPbCl3 perovskite nanocrystals (NCs) with varied sizes were studied in the temperature range of 80 to 320 K. The PL of the three samples are emitted at energies under the corresponding  which indicate that the recombination dynamics in CsPbCl3 NC films is dominated by localized excitons. The lifetime of the three samples showed a trend of increasing initially and then decreasing with the increase of temperature. The longest lifetime of CsPbCl3 NC film for NC398 was 18.17 ns at 200 K, while the values were 13.05 ns and 13.44 ns at 260 K for NC405 and NC407, respectively. The increase in the lifetime can be explained by introducing a charge trapping state or a localized state as a relaxation pathway. The temperature-dependent PL spectra of CsPbCl3 NC films indicate that the three samples themselves have many nonradiative recombination centers, and the optical phonon energies of the three samples are close to the phonon energy obtained previously using Raman spectroscopy.
which indicate that the recombination dynamics in CsPbCl3 NC films is dominated by localized excitons. The lifetime of the three samples showed a trend of increasing initially and then decreasing with the increase of temperature. The longest lifetime of CsPbCl3 NC film for NC398 was 18.17 ns at 200 K, while the values were 13.05 ns and 13.44 ns at 260 K for NC405 and NC407, respectively. The increase in the lifetime can be explained by introducing a charge trapping state or a localized state as a relaxation pathway. The temperature-dependent PL spectra of CsPbCl3 NC films indicate that the three samples themselves have many nonradiative recombination centers, and the optical phonon energies of the three samples are close to the phonon energy obtained previously using Raman spectroscopy.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 11504132, 11704152 and 11774134), Development of Science and Technology of Jilin Province (20190302084GX), and the Thirteenth Five-Year Program for Science and Technology of Education Department of Jilin Province (JJKH20191002KJ and JJKH20180768K).