Abstract
It is established that moderate-to-high doses of ionising radiation increase the risk of subsequent cancer in the exposed individual, but the question arises as to the risk of cancer from higher doses, such as those delivered during radiotherapy, accidents, or deliberate acts of malice. In general, the cumulative dose received during a course of radiation treatment is sufficiently high that it would kill a person if delivered as a single dose to the whole body, but therapeutic doses are carefully fractionated and high/very high doses are generally limited to a small tissue volume under controlled conditions. The very high cumulative doses delivered as fractions during radiation treatment are designed to inactivate diseased cells, but inevitably some healthy cells will also receive high/very high doses. How the doses (ranging from <1 Gy to tens of Gy) received by healthy tissues during radiotherapy affect the risk of second primary cancer is an increasingly important issue to address as more cancer patients survive the disease. Studies show that, except for a turndown for thyroid cancer, a linear dose–response for second primary solid cancers seems to exist over a cumulative gamma radiation dose range of tens of gray, but with a gradient of excess relative risk per Gy that varies with the type of second cancer, and which is notably shallower than that found in the Japanese atomic bomb survivors receiving a single moderate-to-high acute dose. The risk of second primary cancer consequent to high/very high doses of radiation is likely to be due to repopulation of heavily irradiated tissues by surviving stem cells, some of which will have been malignantly transformed by radiation exposure, although the exact mechanism is not known, and various models have been proposed. It is important to understand the mechanisms that lead to the raised risk of second primary cancers consequent to the receipt of high/very high doses, in particular so that the risks associated with novel radiation treatment regimens—for example, intensity modulated radiotherapy and volumetric modulated arc therapy that deliver high doses to the target volume while exposing relatively large volumes of healthy tissue to low/moderate doses, and treatments using protons or heavy ions rather than photons—may be properly assessed.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The risks to health following exposure to ionising radiation are reviewed regularly by various international and national expert groups as evidence is accumulated from studies published in the scientific literature (see, for example, NRC 2006, UNSCEAR 2008, McLean et al 2017, NCRP 2018, Hauptmann et al 2020), and the International Agency for Research on Cancer (IARC) has recognised ionising radiation as an established cause of cancer—it is classified by IARC as a Group 1 carcinogenic agent, 'carcinogenic to humans' (IARC 2000, 2001, 2012, El Ghissassi et al 2009). These scientific reviews are fed into the framework of radiological protection produced by the International Commission on Radiological Protection (ICRP), the most recent general recommendations of which were published in 2007 (ICRP 2007a).
Stochastic health effects are those for which the probability, but not the severity, of the effect varies with the dose of radiation received by the organ/tissue in which the effect originates. The current recommendations of the ICRP (2007a) consider radiation-related stochastic effects to be cancer in the exposed individual and hereditary disease in the subsequently conceived offspring (and their descendants) of the exposed individual. While understanding of the radiobiological mechanisms underlying the complex process through which stochastic effects occur following exposure to radiation is incomplete, available evidence suggests that non-lethal damage to cellular DNA is the principal cause of these effects, and that misrepair of such damage (particularly of localised double-strand breaks) can produce changes in a cell that may eventually lead to malignant neoplastic disease, or hereditary disease if the affected DNA is in a germ cell (Wojcik 2022).
Knowledge of the mechanisms by which radiation interacts with tissues and the biological response to this interaction is insufficient to permit the risks of stochastic effects from radiation exposure to be derived from first principles, so risks must be obtained from epidemiological studies of exposed humans and from in vivo and in vitro laboratory studies. The generalisation of the findings of experimental studies to the everyday experience of humans poses difficulties, so risks are derived principally from epidemiological studies of humans (the species of primary interest) guided by an incomplete understanding of radiobiological mechanisms. However, since epidemiological studies are predominantly observational (i.e. non-experimental) this presents challenges to the proper design, conduct and interpretation of epidemiological studies—the powerful tool of randomisation that makes randomised controlled clinical trials so efficacious is not available to observational epidemiology so that in addition to chance being a possible explanation for a statistical association the roles of bias and confounding must be seriously considered (Hill 2015, Wakeford 2015). As UNSCEAR (2018) has emphasised, 'each [epidemiological] study requires careful and systematic assessment to gauge its contribution to the issue being addressed'.
At present, epidemiological studies of offspring conceived after parental irradiation have not convincingly demonstrated an increase in the risk of hereditary disease. There are indications of exposure-associated increased frequencies of congenital malformations and perinatal deaths in a study of more than 70 000 children born to Japanese survivors of the atomic bombings of Hiroshima and Nagasaki in 1945 (Yamada et al 2021), but the evidence is weak (Lie 2021), and investigations of mutation rates in survivors' children have not demonstrated an effect of parental exposure (e.g. Kodaira et al 2010). Recent studies of the offspring of survivors of childhood and adolescent cancer (e.g. Signorello et al 2012, Winther et al 2012) provide little evidence of adverse heritable genetic effects resulting from treatment, and no increase in rates of germline de novo mutations has been found in the children of Chornobyl clean-up workers (Yeager et al 2021). Epidemiological evidence relating to radiation-associated hereditary disease has been reviewed recently by Boice (2020). Nonetheless, laboratory experiments have demonstrated beyond reasonable doubt that ionising radiation causes gene mutations in many different organisms, and large mouse experiments clearly show that parental irradiation increases the risk of hereditary disease in mammal offspring (UNSCEAR 2001). Consequently, hereditary disease risk estimates are included as a component of the risk of stochastic effects in the ICRP framework of radiological protection (ICRP 2007a). However, the overall risk of stochastic effects, weighted by detriment to health, is now considered to arise predominantly from the risk of cancer in the exposed individual (ICRP 2007a), and this paper will focus upon the radiation-related risk of cancer.
Routine radiological protection generally relates to low-level radiation exposure in the workplace or environment, but high doses may be received as a result of accidents (such as the Chornobyl reactor explosion (UNSCEAR 2011) and the insecure radiation source at Goiânia (IAEA 1988)), deliberate acts of malice (such as the Litvinenko poisoning in London (Harrison et al 2017)) and adventitious high exposures accompanying planned practices (such as the ingestion of substantial quantities of radium-based paint by dial luminisers (Martinez et al 2022)). However, most high doses are received in a medical context and, excluding accidents, intentionally through the use of radiation as a treatment for diseases, in particular, cancer. The ICRP 2007 Recommendations (ICRP 2007a) recognise the need to use radiation for the benefit of the patient as judged by relevant medical practitioners, so the recommendations place emphasis on doing more good than harm through the justification of particular medical procedures and the optimisation of protection.
2. Radiation treatment for cancer
Mettler et al (2020) noted that in the USA in 2016, just over 1 million courses of radiation therapy were administered to about 800 000 patients, and Bryant et al (2017) reported that in 2016 around 3 million (nearly 30% of) cancer survivors in the USA had received radiotherapy. In 2011, a report from the US National Council on Radiation Protection and Measurements (NCRP) noted (NCRP 2011):
As of 2007, there were ∼12 million men and women in the United States with a history of cancer, representing 3.5% of the population. Radiation remains a cornerstone of successful cancer treatment, with 50% of all patients estimated to have received radiation therapy for the management of their cancer.
By 2030, over 22 million cancer survivors are expected to be alive in the USA (Miller et al 2019). As a result of radiation treatment, these patients are assumed to be at some increased risk of second primary cancers (i.e. a subsequent primary cancer that is biologically independent of the first cancer).
During 1973–2000, in nine US Statistics, Epidemiology, and End Results (SEER) cancer registries, cancer survivors were found to have a 14% increased risk of developing a malignant disease compared with the general population (Curtis et al 2006). Curtis et al (2006) concluded,
The overall data from the monograph [(Curtiset al 2006)] suggested that cancer therapy among older adults was not associated with a substantial increase in subsequent cancer risk. In contrast, children and young adults seemed to be especially prone to the carcinogenic effects of intensive radio-chemotherapy regimens (Bhatia 2005, van Leeuwen and Travis 2005).
In a complementary study using data from nine SEER registries for 1975–2013, Morton et al (2017b) concluded that one in five cancer diagnoses involved an individual with a history of cancer. During 1992–2008, from the SEER database, nearly 1 in 12 patients diagnosed with a common cancer developed a second primary malignancy, the most common of which was lung cancer; greater than one-half of patients who experienced two incident cancers died of their second malignancy (Donin et al 2016). Wang et al (2019) used SEER data for 1973–2014 to compare the second primary cancer rate at 20 years after treatment for the first cancer for those patients receiving radiotherapy with the rate for those who did not, and found an overall 14% excess rate in the radiation exposed group, although the excess varied by sex and the site of the second cancer.
Schaapveld et al (2015) showed that the risk of a second primary cancer among survivors of Hodgkin lymphoma was still elevated 35 years or more after treatment by around fourfold when compared with the general population, and the cumulative incidence of a second cancer at 40 years was about 50%. Burt et al (2017) used the SEER database to investigate the incidence of second primary malignancies among women who had been diagnosed with a first breast cancer during 1973–2008, and found that compared to the general US population there was a 20% excess of cancers among patients who had not been treated with radiation, but a 33% excess among those who had been treated with radiation; for the radiotherapy patients, those youngest at exposure displayed the highest excess risk, as did particular sites of second cancers (e.g. oesophagus and leukaemia). The raised risk of second primary cancers among women treated with radiotherapy for first breast cancer was confirmed by a meta-analysis conducted by Grantzau and Overgaard (2016).
The above brief summary indicates that radiation treatment for cancer does confer an excess risk of second primary cancer. Given the frequency of the use of radiotherapy in countries with advanced healthcare systems and the increasing long-term survival of patients treated with radiation, the question arises as to the degree of risk of radiotherapy-related second primary cancers and whether optimal treatment regimens can be identified that maintain the efficacy of the treatment but minimise the risk of second cancers (ICRP 1985, 2007b). To address this question the radiation-related risks from the (very) high doses used to treat cancers must be understood, and the current state of knowledge of these risks will be addressed in this paper.
First, a short history of how radiation risk estimates have been derived from epidemiological studies will be described, concentrating upon groups receiving high doses.
3. Early reports of excess cases of cancer following exposure to radiation
It was in the middle of the twentieth century that human epidemiological evidence began to emerge for radiation exposure increasing the subsequent risk of leukaemia, initially from the experience of radiologists in the USA (Henshaw et al 1944, March 1944, Ulrich 1946, Peller and Pick 1952, Lewis 1963), then from the Japanese survivors of the atomic bombings of Hiroshima and Nagasaki (Folley et al 1952, Lange et al 1954, Moloney and Lange 1954), and then from groups of patients treated with radiotherapy (Simpson et al 1955, Court-Brown and Doll 2007). The evidence that had accumulated by the late-1950s was reviewed by Lewis (1957), Cronkite et al (1960), Hempelmann (1960), Cronkite (1961), who concluded that at high enough doses, radiation could induce leukaemia; Cronkite et al (1960) estimated from reports available in the literature that during 1911–1959, 226 cases of leukaemia could be attributed to radiation exposure.
During the 1950s it was becoming apparent that thyroid cancer was in excess among those receiving x-ray therapy as infants for thymus enlargement (Simpson et al 1955, Simpson and Hempelmann 1957). The 1950s also saw reports of excess cases of cancers of the skin (Petersen 1954), bone (Jones 1953) and of the pharynx and larynx (Goolden 1957, Garrett 1959) following x-ray therapy.
To these groups exposed to predominantly external sources of penetrating energetic photons, i.e. sparsely ionising x-rays and gamma-rays, should be added workers who experienced notable excess rates of cancer as a result of large intakes of radioactive materials that had deposited within the body, including radionuclides emitting short-range, densely ionising alpha particles. Bone and head cancers were notably in excess among radium workers who had ingested high activities of radioisotopes of radium (Aub et al 1952, Fry 1998, Martinez et al 2022), and lung cancer rates were raised among underground hard-rock miners (e.g. uranium miners) who had inhaled high levels of the noble gas radon, principally the radioisotope 222Rn, and its short-lived radioactive decay products (Wagoner et al 1964, 1965). By 1960 it was also becoming apparent that groups of patients who had been injected with the radiographic contrast medium Thorotrast, a colloidal solution containing thorium dioxide, were experiencing excesses of liver cancer as a result of internal irradiation from deposited 232Th and its radioactive decay products (Looney and Colodzin 1956, Baserga et al 1960, Blomberg et al 1963), and that therapeutic injections of 224Ra had produced an excess of cases of bone tumours (Spiess 2002).
Excess rates of leukaemia and other cancers following exposure to radiation were first reported in the mid-twentieth century because investigations had been conducted of sufficiently large groups of people irradiated at sufficiently high levels to achieve a degree of statistical power that was adequate to detect radiation-related increased risks against variations in background rates of cancer incidence or mortality. What was being observed was the effect on cancer risk of moderate and high doses. The Japanese atomic-bomb survivors essentially experienced a uniform whole-body exposure to gamma rays (but with a small component of neutrons), so that all organs/tissues received approximately the same dose, but in most other instances, doses were localised (either through targeted radiotherapy or the heterogeneous internal deposition of radionuclides in the body) such that only certain organs/tissues received moderate and high doses, and the excess cancers that were detected originated in these irradiated organs/tissues.
4. High dose and high dose-rate
The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) defined a 'high dose' in its 1986 Report (UNSCEAR 1986) as a dose of >2 Gy of sparsely ionising radiation or >0.5 Gy of densely ionising radiation. However, the dose-rate at which a dose is received is also of relevance to radiation-related effects, and UNSCEAR (1986) defined a 'high dose-rate' as >0.05 Gy min−1 for all radiations.
The UNSCEAR 1988 Report (UNSCEAR 1988) reaffirmed these definitions of high dose and high dose-rate for sparsely ionising radiation, but added a category of 'very or ultra-high dose' as >10 Gy. For densely ionising radiation, however, UNSCEAR (1988) adopted the corresponding 'dose equivalent' values, in Sv, for defining high dose and high dose-rate, >2 Sv and >0.05 Sv min−1, respectively. The 'quality factors' applied to absorbed doses were those defined by ICRP at that time (e.g. the quality factor for alpha particles was 20), although as emphasised by UNSCEAR (1988), these quality factors, and hence dose equivalents, were defined in the context of radiological protection against low-level exposure, and the factors could not be assumed to be necessarily appropriate for high doses delivered at a high dose-rate. In this paper, we shall follow the UNSCEAR 2012 Report (UNSCEAR 2015) and adopt the definition of a high dose as >1 Gy, supplemented by the definition of a very high dose as >10 Gy as defined in the UNSCEAR 1988 Report (UNSCEAR 1988).
When evaluating stochastic effects consequent to the receipt of high/very high doses of radiation it is important to consider the manner in which the doses are delivered. The risk of cancer arising from a single acute high dose is unlikely to be equal to the same dose received protractedly or as discrete fractions. Low dose-rates will lead to a rate of DNA damage that is capable of repair with high (although not complete) fidelity whereas a high tissue dose received at a high dose-rate will lead to a greater frequency of irreparable DNA damage (leading to cell death) or misrepair (and the possibility of carcinogenic transformation) because of spatially clustered damage wrought by a high level of localised ionisations produced during a short interval.
5. UNSCEAR reviews
UNSCEAR has regularly reviewed the evidence for a raised risk of cancer following exposure to radiation. In its 1964 Report (UNSCEAR 1964), UNSCEAR assessed whether the available evidence was sufficient to make a tentative quantification of radiation-related cancer risk and concluded that 'it is possible, for a few tissues only and mainly in the high dose range, to make estimates of [cancer] risk'. While recognising that following high levels of exposure of certain organs/tissues there was evidence for an increase in risk of cancers such as bone, liver and lung, UNSCEAR considered at that time that risks could only be quantified for leukaemia and thyroid cancer.
UNSCEAR revisited the subject for its 1972 Report (UNSCEAR 1972), i.e. half a century ago, and evidence of increases in cancer risk following exposure to radiation for medical purposes was also reviewed by Hutchison (1972) around the same time. To the previous studies of leukaemia were added studies of patients irradiated to treat metropathia haemorrhagica (Doll and Smith 1968, Alderson and Jackson 1971, Smith and Doll 1976), benign and malignant gynaecological disorders (Wagoner 1984), and cancer of the uterine cervix (Boice and Hutchison 1980). Of interest in respect of the risk from high doses of radiation is the observation of UNSCEAR that an excess of leukaemia was found for moderately exposed sites (<∼3 Gy) but not for heavily irradiated sites (>∼3 Gy). This touches upon an important issue when considering the evidence from high dose studies, namely (UNSCEAR 1972),
It looks more likely that the cell-killing effect of high radiation doses far outweighs their leukaemogenic effect.
In other words, cell killing (or cell sterilisation/inactivation) is an important competing effect to non-lethal carcinogenic modification of cells in tissues receiving high doses of radiation, which reduces the risk of cancer arising in these tissues; at least, at that time, this was true for the haematopoietic system and leukaemia.
The impact of cell killing upon the risk of radiation-induced cancer at high doses was a subject discussed around this time by, among others, Gray (1965) and Mole (1975), the latter emphasising the importance of taking account of cell sterilisation at high doses when considering the dose–response for particular cancer types. Mole (1975) also mentions the influence of repopulation of irradiated tissues by the division of surviving cells, particularly if the exposure is protracted or fractionated rather than a single acute exposure, and this matter will be considered further below. Later, Mole et al (1983) found that the dose–response for acute myeloid leukaemia in male CBA/H mice receiving a single dose of x-rays flattened at around 2–3 Gy and then decreased, a reduction that the authors attributed to a decline in the number of haematopoietic cells surviving the exposure.
UNSCEAR continued to review the scientific literature on the nature of the dose–response for cancer. UNSCEAR (1986) noted that dose–response relationships pass through a maximum before the effect decreases with increasingly higher doses as cell sterilisation becomes an important competitor to carcinogenic transformation (to produce a 'biphasic' dose–response), but that the shape of the dose–response is dependent on cancer type. UNSCEAR (1988) suggested that the variation of the carcinogenic response, R, with the dose, D, might be generally represented by
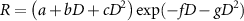
where the exponential term represents the decline in the response due to cell sterilisation at high doses.
6. High/very high doses delivered during radiotherapy
UNSCEAR (1972) introduced an issue of some substance when considering the cancer dose–response at high doses, giving rise to a number of questions: when cell sterilisation plays a significant role in high levels of exposure to radiation, how much is the risk of cancer per unit dose reduced, over what dose range, and how much variation is to be found between different cancer sites? The evidence for the level of carcinogenic effect produced by a single acute dose of radiation essentially delivered uniformly to the whole body (such as experienced by the Japanese atomic bomb survivors) will be limited to a dose range of a few gray because higher doses will be lethal due to damage to the haematopoietic system (an important dose limitation in radiotherapy). However, the fractionated doses used in radiation treatment produce higher cumulative (and generally localised) tissue doses. Following therapeutic doses delivered in fractions, an excess risk of second primary solid cancer is apparent over a tissue dose range of several tens of gray, but with a dose–response for these very high cumulative doses having a shallower slope than that for moderate-to-high doses (Hall 2009). Interpretation is complex because of the risk posed by lower doses remote from the treatment site (e.g. from scattered radiation), but as discussed further below, surviving cells in the vicinity of the highest exposures will proliferate to repopulate tissues denuded by irradiation (Barnett et al 2009), and some of these cells may have undergone malignant transformation. As a consequence, stem cell repopulation will lead to an increased risk of cancer resulting from the very high cumulative tissue doses used in treatment (Lindsay et al 2001, Sachs and Brenner 2005).
The nature of the delivery of a high dose is of considerable importance. As noted above, a high dose received at a low dose-rate over a protracted period would not be expected to produce cell sterilisation to such an extent that a reduction in the risk of cancer would be expected, and results from studies of the Russian Mayak nuclear workers, around 1300 of whom accumulated external gamma doses in excess of 2 Gy over a period of years (Azizova et al 2018), do not suggest a turndown in cancer risk at high doses (Sokolnikov et al 2015, Kuznetsova et al 2016), although the power to discern such an effect may be limited. On the other hand, a single acute whole-body dose of several gray will certainly produce significant cell killing, but to an extent that early death from tissue reactions (haematopoietic failure) might prevent any downturn in cancer risk being observed—without medical intervention, an acute whole-body dose of around 3–5 Gy of gamma radiation will kill some 50% of a normal healthy adult population within 60 days of exposure (ICRP 2007a). For this reason, studies of the Japanese atomic bomb survivors tend to truncate doses at 4 Gy because of doubts over the accuracy of higher doses (Cullings et al 2017), but it is of interest that an earlier study of mortality in the survivors that included higher doses (Pierce et al 1996) did provide evidence of a flattening of the solid cancer and leukaemia dose–responses at high doses (from about 3 Gy), although caution in interpretation is required because this study used DS86 dose estimates rather than the current DS02R1 doses, and because of concerns over the validity of high dose estimates. Schneider and Walsh (2008) used the full dose range (and DS02 doses) for solid cancer incidence in the atomic bomb survivors together with incidence data for second primary solid cancers in Hodgkin lymphoma patients treated with radiation to examine the dose–response at higher doses, and reported a turndown in risk at doses >2 Gy, which they attributed to cell killing. A study of breast cancer incidence after x-ray therapy for acute postpartum mastitis found a linear dose–response in the breast dose range of 0.6–2.5 Gy followed by a flattening in the range 2.5–6.5 Gy and then a reduction in risk in the highest dose group of 6.5–11.5 Gy, which the authors suggested could be the result of cell sterilisation, although they did not exclude this as being a chance effect due to the small number of cases (4) in the highest dose group (Shore et al 1986).
Most interest in the effects of high doses upon the cancer dose–response will centre on those who have undergone radiotherapy because here radiation is being employed as a cell-killer to treat diseased tissue, but under controlled conditions with the clear intention of keeping the patient alive. Outside this medical context, survival following the uncontrolled receipt of doses at the level delivered during radiation treatment would not be possible. Consequently, our principal source of knowledge on the risk of cancer following the receipt of high/very high doses of radiation comes from their use in radiotherapy; early reviews of cancer following external radiation exposure for medical purposes can be found in (Boice 1981, 1988).
A very high dose intentionally delivered to diseased tissue may unavoidably deliver moderate and high doses to healthy tissues fully or partially positioned within the radiation field, but tissues outside the radiation field will also receive a range of doses from radiation leakage and scattering (Purdy 2008). To properly understand the rate of incidence of second primary cancers consequent to radiation therapy, the doses received by the tissues from which the second cancers originate need to be estimated. Given the diversity of the radiotherapy regimens that have been employed and their evolution over time, together with the heterogeneity of distribution of dose between (and within) tissues, the estimation of these doses is far from being straightforward (Schneider and Walsh 2017). Nonetheless, a number of authors (e.g. Stovall et al 2006, 2008, Kry et al 2017, Russell et al 2017, Newhauser et al 2018, Howell et al 2019, Schonfeld et al 2020) have examined methods of reconstructing tissue doses received during radiation therapy, both inside and outside the radiation field, particularly for the purposes of epidemiological studies of survivors. These dosimetry studies are crucial to a proper understanding of the risk of second primary cancers.
UNSCEAR (2000) examined the variation with active bone marrow (ABM) dose of the excess relative risk (ERR) of leukaemia (excluding chronic lymphocytic leukaemia, CLL, now considered to be a form of non-Hodgkin lymphoma (Yu et al 2015, Swerdlow et al 2017)) in the LifeSpan Study (LSS) of Japanese atomic bomb survivors and three groups treated with radiotherapy: British ankylosing spondylitis patients (Weiss et al 1995), international uterine corpus cancer patients (Curtis et al 1994), and international uterine cervix cancer patients (Boice et al 1987); a pooled analysis of leukaemia in the LSS together with the ankylosing spondylitis and cervical cancer patients had previously been reported by Little et al (1999). UNSCEAR (2000) observed that an effect of cell sterilisation in reducing the dose–response at high doses was generally found in the three medically exposed groups, but that the magnitude of the effect varied between the studies, and the uterine cervix and corpus studies also included patients treated with brachytherapy. The interpretation of the findings was complicated by various factors, including the heterogeneity of the ABM dose in the medically irradiated groups and the degree of fractionation and protraction of the exposures, so the proportion of the ABM that received high doses and over what period(s) differed between the studies. Further, Little et al (1999) had found that there were differences in the dose–responses between the three types of leukaemia included in the analyses.
Little (2001a, 2001b) compared the variation with dose of the ERR of cancer incidence and mortality in 65 studies of patients treated with radiotherapy for both malignant and non-malignant diseases with those experienced by comparable matched subsets of the Japanese survivors of the atomic bombings, and found that the relative risks tended to be lower in the medical studies, a finding most marked for leukaemia. Little (2001a, 2001b) concluded that cell sterilisation could largely account for this finding. Blettner and Boice (1991) modelled the risk of leukaemia from the doses received by ABM compartments during radiation treatment for cancer of the uterine cervix and found that cell inactivation by high compartmental doses needed to be included in the model to account for the shape of the dose–response. IARC (2000) also noted the importance of cell inactivation on the flattening of the cancer dose–response at high doses. On the other hand, in their study of leukaemia following radiation treatment for testicular cancer, Travis et al (2000) reported a relative risk for ABM doses ⩾15 Gy of 7.8 (95% CI: 1.1, 79), although the wide confidence interval will be noted.
At the beginning of the twenty-first century, with increasing numbers of patients surviving cancer through advances in treatment, particularly those who had experienced cancer at a young age (Epstein et al 1997), interest grew in the risks of second primary cancers in the survivors and how such risks might be reduced through modifying treatment regimens without impacting upon the efficacy of the treatment (Bhatia and Sklar 2002, Travis 2002, 2006, Hall 2004, Kry et al 2007). Attention began to be focused on cell repopulation following radiotherapy and the implications for second primary cancer risk, with UNSCEAR (2000) noting that the receipt of high doses of radiation, when cell sterilisation becomes important, would be expected to influence the final rate of incidence of second primary cancers not only by initially reducing cell numbers through inactivation, but also by the subsequent mobilisation of quiescent stem cells for tissue repopulation. Some of the repopulating cells may have been malignantly transformed by the irradiation, leading to an increased risk of second primary cancer (Wheldon et al 2000, Lindsay et al 2001).
Of fundamental importance is what constitutes a second primary malignant neoplasm. Warren and Gates (1932) proposed a definition of second primary cancers that remains broadly adopted today:
Each of the tumors must present a definite picture of malignancy, each must be distinct, and the probability of one being a metastasis of the other must be excluded.
Tullis (1942) suggested an additional requirement to exclude tumours with a known tendency for multicentric origin. Among earlier reviews of multiple primary cancers are those of Schottenfeld (1982) and Boice et al (1985a), and studies of the subject have been conducted using the long-established population-based cancer registries in Connecticut and Denmark (Schoenberg 1977, Boice Jr et al 1985b, Boice et al 1986, Storm et al 1986). Later reviews include those of Curtis et al (2006) and Morton et al (2017b) that were mentioned above.
7. Modelling the risk of second primary cancers following fractionated high/very high dose radiotherapy
Sachs and Brenner (2005) observed that studies had shown increasing risks of lung cancer (Gilbert et al 2003) and female breast cancer (Travis et al 2003, van Leeuwen et al 2003) following tissue doses to the site of the tumour ranging over tens of gray received during treatment for Hodgkin lymphoma (figure 1), although the treatment modalities were typical of the 1970s and 1980s (Gilbert et al 2003) so the localised tissue dose estimates have to be uncertain to some extent. They noted that second primary solid cancers after tissue doses this high contradicted the conventional assumption that cell sterilisation dominated the tissue response, and inferred that cell proliferation/repopulation effects need to be taken into account to explain the observed levels of second primary cancers. Sachs and Brenner (2005) proposed a biologically based dose–response model for solid cancer that at high/very high tissue doses incorporated a stem cell repopulation term in addition to taking account of initial cell sterilisation. They concluded that by including the influence of stem cell repopulation, risk estimates are produced that are consistent with the findings for solid cancer after radiation treatment. Shuryak et al (2006) and Little (2007) adapted this repopulation model to address heterogeneity of high doses received by the ABM and the consequent risk of leukaemia—the distribution of the dose received by the ABM during partial-body irradiation is a difficult issue to address because cell sterilisation and repopulation due to high doses in some compartments of the ABM contrast with the leukaemogenic effect of low and moderate doses in other compartments (see, for example, Little et al 2021).
Figure 1. The square markers show the variation with tissue absorbed dose at the tumour site of the excess relative risks of (A) lung cancer and (B) breast cancer following radiation treatment for Hodgkin lymphoma. The dashed lines show the results of the 'standard model' as applied to data for the Japanese atomic bomb survivors (diamond markers). Reproduced with permission from Sachs and Brenner (2005), Copyright (2005) National Academy of Sciences, U.S.A.
Download figure:
Standard image High-resolution imageModelling of the effects of high doses in terms of initiation, inactivation and repopulation in the short term, and promotion, clonal expansion and transformation in the long term was developed further by Shuryak et al (2009a, 2009b, 2011), Ng and Shuryak (2015), who emphasised the importance of developing an appropriate model of the relevant biological mechanisms to understand the impact on second primary cancer risk of novel radiation treatments. Other mechanistic models have also been developed (Schneider 2009, Schneider et al 2011a, 2011b) and applied to predicting the risk of second primary cancer for different radiotherapy modalities by Timlin et al (2021). Schneider and Schäfer (2012) considered the role of proliferative stress and inflammation-based carcinogenesis at very high tissue doses and included these aspects in a model of second primary cancer following fractionated therapeutic doses. However, our intention is not to examine mechanisms in detail, but rather to provide a description of the risk of second primary cancer as set out by the most relevant epidemiological studies of the subject.
8. Risk of second primary cancers in survivors of childhood cancer
Survivors of childhood cancer are of particular interest in terms of their health in later life because >80% of children now survive cancer, the population of childhood cancer survivors is growing and exceeds half a million people in the USA alone, and survivors have decades of life over which any increased risk may be expressed (Nottage et al 2011, Reulen et al 2011, Morton et al 2014, Robison and Hudson 2014, Turcotte et al 2015, Winther et al 2015, Bhakta et al 2017, Demoor-Goldschmidt and de Vathaire 2019, Erdmann et al 2021). Indeed, multiple primary cancers have been reported in aging survivors of childhood cancer (Armstrong et al 2011). Further, from the experience of children exposed to lower levels of radiation, it might be expected that, in general, the cancer risk per unit tissue dose is greater at younger ages at exposure (UNSCEAR 2013). In a review of the evidence available in the early-1980s, Coleman (1982a, 1982b) drew attention to the risk of second primary cancer following radiotherapy at a young age, and discussed the level of such risk, particularly in the context of the impact of cell killing by high doses. A meta-analysis of the findings of 26 studies of second primary cancer after radiation treatment for childhood cancer reported an ERR/Gy of 0.60 (95% CI: 0.31, 1.15), although highly significant heterogeneity of study findings was found, probably due, at least in large part, to all types of second cancer being combined in the analysis (Doi et al 2011).
Olsen et al (2009) investigated the lifelong incidence of second primary cancers in nearly 48 000 survivors of childhood cancer diagnosed during 1943–2005 in the Nordic countries. Overall, the incidence rate was increased threefold compared to the general population, and the increase was discernible even among survivors approaching an attained age of 70 years. Inskip et al (2016) studied the incidence of second primary solid cancers in just over 12 250 children diagnosed with cancer in North America during 1970–1986, who were the group of >85% of patients who had survived >5 years. Tissue doses were reconstructed and linear dose–responses over the dose range evaluated, doses up to 50 Gy, were found for all solid cancers except thyroid (which showed a downturn at 15–20 Gy), with different second cancers displaying various ERR/Gy slopes (figure 2). However, Inskip et al (2016) caution that generalising findings for childhood cancer survivors to the wider population may not be valid because the survivors may be predisposed to developing another primary cancer (Wang et al 2018). Nonetheless, Inskip et al (2016) drew the important conclusion that their results show that
treatment effects in most instances predominate over inherent susceptibility factors related to type of first cancer, and that meaningful inferences can be drawn about radiation effects from studies of new cancers among persons with different types of first cancer.
Figure 2. Fitted radiation dose–response by type of second primary solid cancer, as derived from models based on data from the Childhood Cancer Survivor Study of North America. Reproduced from Inskip et al (2016), Copyright (2016), with permission from Elsevier.
Download figure:
Standard image High-resolution imageArmstrong et al (2016) and Turcotte et al (2017, 2018) reported that although the risk of second primary cancer remained increased among 5-year survivors of childhood cancer diagnosed in the 1990s, the risk was lower when compared with those diagnosed in the 1970s, which they attributed to a reduction in therapeutic radiation dose.
Studies of breast cancer in survivors of childhood cancer have provided clear evidence of an increased risk at breast doses ranging over tens of gray (Inskip et al 2009), and a detailed examination by Schonfeld et al (2020) of the breast doses delivered by radiotherapy confirmed this finding. Travis et al (2005) found similar results for young women irradiated to treat Hodgkin lymphoma, as did Moskowitz et al (2014) in their study of breast cancer in women who had received breast doses in excess of 10 Gy during chest radiation therapy for childhood cancer; Moskowitz et al (2014) reported that the magnitude of the breast cancer risk by the age of 50 years in survivors of childhood cancer was comparable to that of BRCA mutation carriers. Veiga et al (2019) found no significant departure from a linear dose–response for breast doses up to 50 Gy received during treatment for childhood cancer. Hodgson et al (2017) reviewed the risk of breast cancer following treatment for cancer at a young age, including the risk from radiation treatment. They noted that an 'appropriate inference' from historical studies would be 'that increasing breast doses from 5 to 40 Gy are increasingly carcinogenic', but cautioned that factors other than dose are important in determining risk, such as volume of breast tissue exposed, together with constitutional factors, chemotherapy and genetic predisposition.
Taylor et al (2010) studied the incidence of brain and other central nervous system (CNS) tumours following radiation treatment for childhood cancer and found that the risk notably increased linearly with tissue dose over a range up to 50 Gy. An earlier study of tumours of the brain and neural system following irradiation in childhood to treat ringworm of the scalp found a 'strong dose-response' over a brain dose range of 1–6 Gy (Ron et al 1988). Lorenz et al (2018) reviewed studies of thyroid cancer following radiation treatment for childhood cancer and found 'conclusive evidence' that radiotherapy leads to an increased risk of second primary thyroid cancer.
With a different and somewhat simplistic approach, Diallo et al (2009) investigated the doses at the site of the second primary solid cancer that had been received during radiation treatment for a first primary cancer during childhood. Of the 115 second primary cancers, 14 (10 sarcomas) were 'clearly-in-beam' (median dose, 37 Gy), 76 (36 sarcomas) were bordering the beam (median dose, 20 Gy), while the remaining 25 (6 sarcomas) were in locations distant from the beam (median dose, 0.3 Gy). Tucker et al (1987) had earlier identified a large ERR of bone sarcomas in those who had received very high bone doses during radiotherapy for cancer in childhood. Similarly, Schwartz et al (2014) found a pronounced increase of bone sarcomas among patients who had been treated with radiation for a childhood solid cancer. In a nested case-control study of 190 second primary solid cancers, Hennewig et al (2014) examined the effect of dose received during radiation treatment for childhood cancer on the risk of the second cancer. They found that 147 patients had received radiotherapy and that irradiation had increased the risk of a second primary solid cancer in the region of the target tissues (median dose, 25 Gy) by ∼70% per 10 Gy, but found little effect of doses received by regions of the body adjacent to the site of the second cancer or distant from the site.
Recently, Schonfeld et al (2021) examined the long-term risk of second primary cancers among retinoblastoma patients from two major medical centres in New York and Boston, of whom 1128 and 924 had survived hereditary and nonhereditary retinoblastoma, respectively, with 87% of the former and 20% of the latter known to have received radiotherapy. Observed numbers of second cancers were compared with the numbers of cases expected from SEER rates. The increased risks of bone and soft tissue sarcomas and melanoma after hereditary retinoblastoma are well established (Morton et al 2017b), and Schonfeld et al (2021) confirmed this finding, but they also found significantly increased risks for a limited number of epithelial tumours: CNS, pineoblastoma, nasal and oral cavities, and breast. Of note are the substantially increased risks for tumours of the CNS, nasal and oral cavities and of pineoblastoma, because all observed tumours at these sites occurred among patients who had received radiation treatment and the sites were in or near the radiation field. No significantly increased risk of second primary cancers was found for survivors of nonhereditary retinoblastoma.
Allodji et al (2021) conducted a pooled case-control study of 147 cases of second primary leukaemia among survivors of childhood cancer diagnosed during 1930–2000 in one of six countries; two-thirds of the cases were of acute myeloid leukaemia. Doses to the ABM were reconstructed. Overall, the risk of second primary leukaemia was associated with radiotherapy (primarily during the first decade after treatment), but the association was much stronger among those that had not also been treated with chemotherapy: using a linear dose–response model, the excess odds ratio per unit absorbed dose (EOR/Gy) was significantly elevated for those not receiving chemotherapy, 1.55 (95% CI: 0.14, 14.3), but not for those also receiving chemotherapy, 0.02 (95% CI: −0.01, 0.09), and the difference in EOR/Gy was significant. There was a suggestion that the risk from radiotherapy was somewhat larger for ABM doses <12 Gy than >12 Gy in the absence of chemotherapy, but not when treatment also included chemotherapy, although small numbers for those treated with radiotherapy alone limit interpretation. The authors opined that the strongly elevated risk of leukaemia associated with treatment with chemotherapy alone may have influenced the findings for radiotherapy; the potential interaction between radiation and chemotherapy has implications for leukaemogenic mechanisms.
The challenges of modelling these subtle biological mechanisms using observational data need to be considered when interpreting results: retrospective estimation of tissue doses (as discussed above), development of dose metrics that are capable of handling exposures with steep dose gradients, competing mortality or prophylactic surgery, and interaction of radiation with chemotherapy (e.g. see the discussion in the preceding paragraph) or radiotherapy to other body parts. As an example, Inskip et al (2009) demonstrated that radiation-related breast cancer risk per unit breast dose was substantially reduced among female childhood cancer survivors who received substantial doses to the ovaries, which was likely to have been caused by a reduction in the stimulating effects of ovarian hormones on breast cells with radiation damage.
9. Risk of second primary cancers after radiotherapy
In the international study of second primary cancer following radiation treatment for cancer of the uterine cervix, Boice et al (1988) found raised risks for some heavily irradiated sites (e.g. rectum and bladder), but not others (e.g. colon); lower (but still high) doses produced excess risks of stomach cancer and leukaemia while moderate doses to the breast and thyroid were not associated with a raised risk. These findings were largely borne out in a later study with longer follow-up by Chaturvedi et al (2007), and an excess risk of colon cancer could also be discerned. A study of second primary breast cancer in the contralateral breast following first breast cancer (Boice et al 1992) found an elevated risk for women treated while <40 years of age who had received a dose >1.0 Gy to the specific quadrant of the contralateral breast (Stovall et al 2008); the mean dose to the specific quadrant was 1.1 Gy with a range up to 6.2 Gy, which is lower than some other studies of second breast cancer.
The risk of second primary cancers following radiation treatment for cancer was comprehensively reviewed in NCRP Report No. 170 (NCRP 2011), the findings of which were summarised by Travis et al (2012, 2014). The results of studies of various second primary cancer sites following radiation treatment were tabulated and discussed in the report, and dose–responses presented. The variety of dose–response shapes was noted, and the contrast between the steep increase in the risk of leukaemia in the Japanese atomic bomb survivors receiving low-to-moderate ABM doses and the increased but rather flat (over a mean ABM dose range up to 10 Gy) leukaemia risk in different groups of patients irradiated therapeutically was commented upon. The NCRP review stressed the importance of an ongoing evaluation of risks through the continued follow-up of survivors of older treatment regimens and the application of these findings to new radiation modalities and techniques as they are developed, complemented by enhanced knowledge of underlying radiobiological mechanisms. A number of recommendations were made for studies to improve the understanding of the risk of second primary cancers following radiotherapy, particularly dose–response relationships and their modification by other factors. Broader issues involving the risk of second primary cancers were reviewed by Travis et al (2013), Choi et al (2014) and Black et al (2014) emphasised the need to take into account those factors that increase the risk of second primary cancers but are not related to treatment; pooled studies are required to provide the large numbers of cases needed to obtain meaningful results.
Berrington de Gonzalez et al (2011) identified adults who had developed one of 15 types of first primary solid cancers routinely treated with radiotherapy, recorded in one of nine US SEER cancer registries as being diagnosed during 1973–2002. In 5-year survivors, the proportion of second primary solid cancers among patients who had received radiotherapy was compared with the equivalent proportion among those who had not been treated with radiation. For each of the first cancer sites the relative risk (RR) exceeded 1.0, and Berrington de Gonzalez et al (2011) concluded that around 8% of second primary cancers could be attributed to the radiation treatment. Of interest is that the RR tended to be greatest for those tissues for which the dose typically exceeded 5 Gy.
In a subsequent study, Berrington de Gonzalez et al (2013) reviewed the epidemiological evidence for the risk of second primary solid cancers in 11 tissues that had received doses >5 Gy during radiotherapy. Apart from thyroid cancer after radiation treatment in childhood, which showed a downturn at doses >20 Gy—a finding earlier reported by Bhatti et al (2010) (the basis of the thyroid cancer plot shown in figure 2) and later confirmed by de Vathaire et al (2015) and by a pooled analysis of 12 studies by Veiga et al (2016)—the second cancers exhibited linear dose–responses over a high/very high dose range of tens of Gy, but the ERR/Gy slopes were markedly lower (by factors of 5–10) than those found in the LSS at doses <2 Gy. Berrington de Gonzalez et al (2013) emphasised the importance of obtaining a better understanding of the risk of second primary cancers following fractionated high tissue doses received during radiation treatment in the past to inform on the risks resulting from the continuing improvements in radiotherapy techniques—it would take many years of follow-up to obtain direct evidence of the risk of new modalities, so a better knowledge of biological mechanisms at high doses is essential.
Ng and Shuryak (2015) and Kamran et al (2016) examined factors that affect the risk of second primary cancers after high therapeutic doses of radiation. They identified as important the organ/tissue that is irradiated, the dose received and the volume of tissue irradiated, the age of the patient when treated, concomitant chemotherapy use, and genetic risk factors, among other factors. Age-at-exposure has a strong influence on the risk of second primary cancers, with younger patients having the highest risk.
Gilbert et al (2017) examined the risk of stomach cancer following fractionated high/very high dose radiotherapy to treat Hodgkin lymphoma, testicular cancer or cervical cancer by pooling data from three nested case-control studies of five year survivors. Cumulative doses to the site of the tumour in the stomach (and equivalent for matched controls) were reconstructed; the overall mean dose was 10.3 Gy. A linear dose–response was found over a dose range of tens of Gy with a highly significantly positive slope and no evidence of a decrease in the slope at doses in excess of 35 Gy (figure 3). The pooled EOR/Gy was 0.091 (95% CI: 0.036, 0.20), much less than that found for stomach cancer in the atomic bomb survivors. Of interest is that there was a significant increase of EOR/Gy with time since exposure, which was not observed in the LSS. The authors concluded that these findings emphasise the need for direct studies of the incidence of second primary cancers in patients treated with fractionated high dose radiotherapy.
Figure 3. Dose–response for stomach cancer following radiation treatment for Hodgkin lymphoma, testicular cancer or cervical cancer. The dose is the cumulative dose received by the site of the stomach tumour from the radiation treatment. Excess odds ratios and 95% confidence intervals, with fitted linear model. Reproduced with permission from Gilbert et al (2017). © 2022 Radiation Research Society.
Download figure:
Standard image High-resolution imagePredicting how the risk of second primary cancers varies with dose is a complex process, not least because of the variety of treatment protocols that have been adopted over the years (e.g. the degree of fractionation of the cumulative dose delivered to diseased tissues and how radiotherapy has been used with chemotherapy). Observational studies on the late effects of radiotherapy are, of necessity, based on patients treated many years, often decades, in the past. However, treatment protocols have evolved (as sometimes have the radiations employed) leading to uncertainties in the doses received by the pertinent tissues of patients included in the studies and in the resulting risk estimates, and in their application to modern treatment regimens. Compared to classic external beam radiotherapy, intensity modulated radiotherapy and volumetric modulated arc therapy irradiate the target volume with (very) high doses while exposing relatively large volumes of healthy tissues to low/moderate doses. Proton or heavy ion therapy (as opposed to photon irradiation) can conform more precisely to the treatment volume thereby reducing the exposure of healthy tissue (Durante 2021), although other issues can be introduced, such as secondary neutrons produced by proton interactions with nuclei (Paganetti 2012). To this must be added the differing responses to high radiation doses of the various tissues involved in the treatment, and risk modification by factors such as the variation of cell repopulation with age-at-exposure.
Journy et al (2019) conducted a review of clinical and epidemiological studies of second primary cancer following radiotherapy, with the aim of comparing the risks in, or close to, target volumes with those in remaining tissues. They found evidence for an increased risk of second cancers in the tissues that had been the target (or in the immediate vicinity of the target) of the treatment, but insufficient information was available to obtain a direct estimate of risk in tissues away from the irradiation target that had received low-to-moderate doses. Cancer mortality after radiotherapy for benign gynaecological disorders was studied by Sakata et al (2012), who found evidence that for heavily irradiated sites (having reconstructed median tissue-specific doses in the range 1–10 Gy), radiation-related excess risks existed for cancers of the bladder, rectum and ovary (and suggested for colon cancer), and for leukaemia (excluding CLL). Other cancers originating in tissues with lower exposures showed little evidence for an excess risk related to radiation exposure. In a study of the site of a second primary cancer in relation to the radiation treatment field, Dörr and Herrmann (2002) found that nearly 60% of second cancers developed within tissues corresponding to the 'penumbra' of the initial radiotherapy volume and receiving a dose <6 Gy, while about 35% developed at doses between 10 and 30 Gy. Rubino et al (2005) conducted a nested case-control study of 14 breast cancer patients who subsequently developed a sarcoma and found that the site of the sarcoma was always located in the radiation field; they reported that the risk of sarcoma was a significant factor of ∼30 higher for doses ⩾45 Gy than for doses <15 Gy at the site of the sarcoma.
In an extensive review of evidence available in 2006, both epidemiological and experimental, relating to subsequent cancer in patients treated with radiation, Suit et al (2007) concluded from an analysis of 14 groups receiving radiotherapy that an increased risk exists over a wide range of doses, but that the magnitude of the increased risk varies between the site of the cancer.
Simonetto et al (2021) studied the risk of second primary cancers among women treated with radiation therapy for a first breast cancer, and paid particular attention to the range of doses received by tissues during the treatment. They generated risk models for lung cancer, breast cancer and leukaemia using LSS data for tissues receiving low-to-moderate doses and the results of a meta-analysis of studies of therapeutic exposures for tissues receiving high or very high doses, and interpolated between these two dose ranges. The high/very high dose model for lung cancer was based on six studies and the ERR was linear in dose with a slope (ERR/Gy) of 0.16 (95% CI: 0.05, 0.27), while that for breast cancer was based on eight studies and produced a linear dose–response with ERR/Gy = 0.18 (95% CI: 0.01, 0.38). However, there was significant heterogeneity between the eight study results for breast cancer and it is of note that the two studies of contralateral breast cancer following treatment for a first breast cancer (Storm et al 1992, Stovall et al 2008) did not show an increased risk, and that the highest and most statistically significant ERR/Gy was for women whose breasts were periodically exposed to low doses while they were being monitored for tuberculosis treatment (Boice et al 1991) whose cumulative risk would not be materially affected by cell sterilisation. Simonetto et al (2021) found that a formal meta-analysis was not feasible for leukaemia, so their high/very high dose model was based upon a selection of models from four studies, which produced a variation of ERR with dose that was rather flat but very uncertain, with central values of ERR ranging from 0.11 to 1.6 depending on the model adopted.
Of interest with respect to the radiation-related risk of second primary cancers is the study of Li et al (2010) of just over 1000 second primary cancers among approximately 14 000 members of the LSS cohort of Japanese atomic bomb survivors who had been diagnosed with a first primary cancer; the tissue doses used in this study were those received during the bombings and not as a result of radiation treatment for the first cancer. For solid cancer, the ERR/Gy estimates for first and second primary cancers were 0.65 (95% CI: 0.57, 0.74) and 0.56 (95% CI: 0.33, 0.80), respectively, while those for leukaemia were 2.65 (95% CI: 1.78, 3.78) and 3.65 (95% CI: 0.96, 10.70), respectively. The compatibility of the ERR/Gy estimates for first and second primary cancers will be noted, and those first solid cancers that showed the largest response to radiation (lung, colon, female breast, thyroid and bladder) were also the second primary cancer types having the largest responses; this compatibility extended to survivors who received doses >1 Gy during the bombings. As remarked by the authors, the ERR/Gy estimates were not altered discernibly by the treatment of the first cancers with radiotherapy or chemotherapy. As has been pointed out above, the slope of the dose–response found in the LSS cohort for both first and second primary cancers is steeper than that observed in studies of high and very high doses received from radiotherapy.
Studies of molecular and genetic markers and genome-wide association studies (GWAS) offer the promise of better understanding the mechanistic processes involved in therapy-related cancer (Morton et al 2017b); Rutten and Badie (2021) have provided a précis of the current status of radiation biomarkers. It is established that chromosome translocation frequencies in peripheral blood lymphocytes are a reflection of dose to the ABM if measured in stable cells (Tawn and Whitehouse 2003), and micronuclei frequencies in solid cancer cells following radiotherapy have recently been reported by Kobayashi et al (2020). Behjati et al (2016), Kocakavuk et al (2021), Morton et al (2020) and Haddy et al (2014) have investigated various mutational signatures of the involvement of radiation in second primary cancer, while Morton et al (2017a) conducted a GWAS and Opstal-van Winden et al (2019) a study of single-nucleotide polymorphisms in investigations of the susceptibility of patients to radiation-related breast cancer. Qin et al (2020) studied the potential interaction between cancer treatment and pathogenic germline mutations in DNA repair genes that predispose to the development of cancer by performing whole-genome sequencing on DNA from 4402 childhood cancer survivors and relating the risks of second primary cancers to radiotherapy/chemotherapy exposures in combination with the mutation status of the survivors; half the survivors were treated with radiotherapy. Mutations in homologous recombination genes were significantly associated with an increased rate of second primary female breast cancer, particularly among survivors who had received chest doses ⩾20 Gy, and mutations in nucleotide excision repair genes were associated with second primary thyroid cancer among survivors who had received neck doses ⩾30 Gy. These and similar studies of the fundamental aspects of carcinogenesis at the molecular level offer an insight into the patterns of risk observed in epidemiological studies of second primary cancer following radiotherapy. This approach is likely to shine a light on the optimisation of treatment efficacy against consequent risk, and on the requirement of surveillance of those most at risk of a second primary cancer. However, there is some way to go yet.
10. Concluding remarks
In the absence of medical intervention, uniform whole-body exposure to gamma radiation presents a ∼50% risk of early death (that is, within 60 d of exposure) from tissue reactions (haematopoietic failure) in a healthy adult population receiving an acute dose of 3–5 Gy (ICRP 2007a). This is due to ionising radiation being a rather effective cell killer—hence the efficacy of radiotherapy—but dead cells cannot develop into a malignant neoplasm, so cell sterilisation competes with non-lethal carcinogenic modification of cells in terms of overall effect at the tissue level. There is some evidence from the Japanese atomic bomb survivors of cell sterilisation producing a flattening of the dose–response for cancer at doses above 3 Gy, but this reduction in the dose–response slope is unclear because of dosimetry uncertainties at high estimated doses.
For partial-body irradiation, cell inactivation occurs in those tissues receiving high doses, but provided the exposed individual survives the early tissue reactions produced by the localised high doses, stem cell repopulation of heavily irradiated tissues is an important subsequent effect in tissue recovery. The repopulation of heavily irradiated tissues by surviving stem cells is also fundamental to the radiation treatment strategy of dose fractionation that permits a high cumulative dose to be delivered to diseased cells while allowing tissue recovery and survival of the patient. However, some of the proliferating stem cells may have undergone carcinogenic transformation through exposure to radiation, leading to an increased risk of second primary cancers.
The risk of second primary cancers following cumulative high doses varies with the tissue that is exposed. Except for a turndown in the risk of cancer of the thyroid gland, the risk of second primary solid cancers looks to increase linearly with increasing cumulative tissue dose over a dose range of tens of gray, but the ERR/Gy varies with cancer type and is notably less than that experienced by the Japanese atomic bomb survivors who received moderate-to-high whole-body doses. The ERR/Gy is greater for those exposed at a younger age, and there are other factors that modify the excess risk.
Survival from cancer continues to improve, and radiotherapy plays an important role in this success. However, it is clear that the (very) high tissue doses received during radiation treatment also lead to an increased risk of second primary cancers. Radiotherapy regimens evolve, but the epidemiological studies of survivors of necessity involve those treated with older modalities. As a consequence, the proper interpretation of the findings from cancer survivor studies is vital for the accurate application of these findings to current treatment regimens (see, for example, DeLaney et al 2020). Ultimately, this must mean a detailed understanding of mechanisms, and this is the goal of research. Nonetheless, recognition of the risk of second primary cancers in survivors of a first cancer has led to surveillance for late effects in patients surviving cancer at a young age (see, for example, Kremer et al 2013).
When radiation exposure is deliberate and justified as in medicine, the fundamental principle is to do more good than harm (ICRP 2007a, 2007b). Curing patients using radiotherapy is obviously doing good, but optimisation is key: the probability of achieving a cure is maximised while the risk arising from the exposure is minimised. Getting that optimisation right continues to be the challenge facing those designing treatments that deliver high radiation doses to patients.
Acknowledgments
We wish to acknowledge the constructive comments received from the anonymous reviewers, which significantly improved the paper.
R W is a member of the Technical Working Party of the UK Compensation Scheme for Radiation-linked Diseases, www.csrld.org.uk/, for which he receives a consultancy fee; the CSRLD was not involved in any way in the writing of this paper. No funding was received by either author for the writing of this paper.




