Abstract
18F has been the most widely used radionuclide in positron emission tomography (PET) facilities over the last few decades. However, increased interest in novel PET tracers, theranostics and immuno-PET has led to significant growth in clinically used positron-emitting radionuclides. The decay schemes of each of these radioisotopes are markedly different from 18F, with different endpoint energies for the emitted positrons and, in some cases, additional high energy gamma radiation. This has implications for the occupational exposure of personnel involved in the manipulation and dispensing of PET radiopharmaceuticals. The EGSnrc Monte Carlo simulation software was used to estimate the doses to extremities in contact with unshielded and shielded syringes containing 64Cu, 18F, 11C, 13N, 15O, 68Ga and 89Zr, respectively. Dose rates at various distances from the syringe were also modelled, with dose rates reported in terms of eye (Hp(3)), skin equivalent (Hp(0.07)) and deep (Hp(10)) doses. The composition and geometry of the simulated syringe shields were based on a selection of commercially available PET shields. Experimental dose rate measurements were performed for validation purposes where possible. Contact skin dose rates for all isotopes, except for 64Cu, were found to be higher than 18F for the unshielded syringe. The addition of a shield resulted in approximately equal contact skin dose rates for nearly all isotopes, for each shield type, with the exception of 89Zr which was notably higher. Dose rate constants (µGy/MBq.hr) for a range of PET isotopes and shields are presented and their significance discussed.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
18F has been the most widely used radionuclide in positron emission tomography (PET) facilities over the last few decades. However, increased interest in novel PET tracers, theranostics and immuno-PET over the past few years has led to the growth in clinically used positron-emitting radionuclides [1]. In particular Gallium (68Ge/68Ga) generators are becoming ever more prevalent in PET/CT facilities for gallium-labelled imaging of prostate cancer and neuroendocrine tumours [2, 3], while other radioisotopes such as 11C, 13N, 15O, 64Cu, 89Zr and 82Rb have also demonstrated their potential for more widespread clinical use [1]. The decay schemes of each of these radioisotopes are markedly different from 18F, with different endpoint energy for the emitted positrons and, in some cases, additional high energy gamma radiation [4, 5] (table 1). This has implications for the occupational exposure of nuclear medicine personnel involved in the manipulation and dispensing of radiopharmaceuticals [6].
Table 1. Decay characteristics of a range of PET isotopes [4, 5].
| Isotope | Half-life (min) | Decay | Beta Emax (keV) (%) | Beta Emean (keV) | Gamma keV (%) |
|---|---|---|---|---|---|
| 18F | 110.0 | β+ | 634 (97%) | 250 | 511 (194%) |
| 68Ga | 67.8 | β+ | 1899 (88%) | 836 | 511 (178%) |
| 1077 (3%) | |||||
| 11C | 20.4 | β+ | 960 (100%) | 386 | 511 (200%) |
| 13N | 10.0 | β+ | 1199 (100%) | 493 | 511 (200%) |
| 15O | 2.0 | β+ | 1735 (100%) | 737 | 511 (200%) |
| 64Cu | 12.7 h | β− | 579 (38%) | 191 | 511 (35%) |
| β+ | 653 (18%) | 278 | — | ||
| 89Zr | 78.4 h | β+ | 902 (23%) | 396 | 511 (46%) |
| 909 (99%) | |||||
| 82Rb | 1.3 | β+ | 3381 (81.8%) | 1536 | 511 (191%) |
| β+ | 2605 (13%) | 1168 | 777 (15%) |
Occupational doses in the form of skin dose (Hp(0.07)) and deep dose (Hp(10)) are typically monitored for PET and nuclear medicine personnel [7]. In order to reduce occupational exposure, the use of commercially available syringe shields is recommended [8]. These shields, originally designed to attenuate the 511 keV gamma photons of 18F, are now routinely used with a range of other PET radionuclides despite the fact that, in general, these nuclides have more complex decay schemes than the pure positron (β+) emitting 18F. Indeed, the emission of higher energy positrons, γ-gamma rays and beta (β−) particles can all have significant implications for occupational exposure. Thus, careful consideration must be paid to use of shielding designed specifically for 18F when dealing with other PET radionuclides.
Calculations based on the decay characteristics of 68Ga and 18F had found that the skin dose rate (mSv/MBq.hr) due to direct contact with an unshielded 5 ml syringe is approximately 11 times higher for 68Ga than 18F [6, 9]. The positrons from 18F have a lower energy and range than those of 68Ga and, thus, many of the 18F positrons do not contribute to skin dose, as they are stopped by the wall of the syringe. The contact skin dose rates for 11C, 13N and 15O have been reported to be approximately 2, 4 and 11 times higher than that of 18F, respectively [9]. 64Cu, with its lower positron energies and less frequent emissions, has a contact dose rate approximately equal to 20% of that of 18F. The above calculations, however, are based on simple and limited geometries, which may not reflect the actual dose rates received in clinical practice.
The International Commission on Radiation Protection (ICRP) guidelines on eye lens dose monitoring [10] state that monitoring of the beta radiation dose contribution to the eye lens (Hp(3)) for isotopes with maximum energies >700 keV may be required if the shielding used is not sufficient to absorb the beta radiation completely. Beta particles with maximum energies in excess of 700 keV can penetrate the lens of the eye resulting in an exposure. While eye dose monitoring is not routinely performed in PET departments where 18F is the only radionuclide employed, for higher energy positron-emitting radionuclides there is the potential for increased eye doses, particularly in situations where staff are exposed to unshielded sources, so eye dose monitoring may be required.
The literature has reported Monte Carlo simulations of skin dose rates (Hp(0.07)) and deep dose rates (Hp(10)) for various situations where an operator is in direct contact or distant from some unshielded PET radionuclide sources [11, 12]. However, to date, skin, eye and deep doses from shielded PET radionuclide syringe sources have not yet been described. Accordingly, the aim of this study was to address some of these gaps by establishing, through Monte Carlo simulation and experimental measurement, Hp(0.07), Hp(10) and Hp(3) doses received from shielded and unshielded syringes containing a range of PET radionuclides. As this study specifically assesses the radiation exposure from positron-emitting radionuclide solutions in syringes, the results are primarily relevant during dispensing and administration tasks.
2. Materials and methods
A number of different Monte Carlo codes have been employed for radiation transport applications, including GEANT4, MCNP5, Penelope and EGNnrc. In this work, Monte Carlo simulations were carried out with EGSnrc version v2019a, National Research Council Canada [13]. EGSnrc is a software toolkit that performs Monte Carlo simulation of ionising radiation transport through matter. It models the propagation of photons, electrons and positrons with a range of kinetic energies in homogeneous materials. EGSnrc is widely used in medical physics applications, including modelling of photon and electron beams in linear accelerators, dose determination, detector response characterisation and assessment of radiation shielding.
The sources were simulated using the EGSnrc radionuclide source module according to their evaluated nuclear structure data file [14]. Electrons and photons were followed down to a kinetic energy of 10 keV. All results were normalised to a time-integrated activity of 1 MBq.hr. The number of histories used in each simulation was chosen to ensure relative statistical uncertainties lower than 3% (comparable to typical uncertainties in radionuclide activity measurements and significantly lower than the tolerances of most commonly used survey meters), unless otherwise noted.
2.1. Validation of EGSnrc
In order to first validate the suitability of EGSnrc and its radionuclide source module for dose evaluations Monte Carlo simulations of radioactive aqueous solutions using the GEANT-4 code [15], as described in Amato et al [11] were reproduced making use of EGSnrc. Comparison of the results not only provided confirmation of the suitability of EGSnrc for this type of simulation, but also allowed a comparison to be made between two different radiation transport algorithms. The modelled geometry consisted of a radioactive water solution contained within a cylindrical receptacle of internal diameter of 1 cm and volume of 2.5 cm3. The cylindrical source was then placed at the centre of a spherical shell of soft tissue (figure 1(a)). The soft tissue was considered to be composed of ICRP 4 component soft tissue (ρ = 1.03 g cm−3; 10.12% H, 11.1% C, 2.6% N and 76.18% O—percentages per weight) [16]. The soft tissue shell was 10 cm thick and had an inner radius varying from 10 to 100 cm, in order to evaluate doses at 10, 30, 50 and 100 cm distance from the source. Two scoring volumes were defined inside the absorber. A spherical shell of 10 µm thickness was centred at a depth of 70 µm (65–75 µm) to evaluate Hp(0.07). A 2nd spherical shell of 1 mm thickness was centred at a depth of 10 mm (9.5–10.5 mm) to evaluate Hp(10). Dry air (ρ = 1.20479 × 10−3 g cm−3) filled the space between the cylindrical source and the spherical shells. The average per cent deviation of EGSnrc from Amato's work was found to be −0.2% and 0.8% (all isotopes <±3%) for the Hp(10) deep dose at 10 cm and 30 cm, respectively, from the radionuclide source. In the case of the Hp(0.07) skin doses, the average per cent deviation of EGSnrc with respect to Amato was −6.9% and −5.2% at 10 cm and 30 cm, respectively, from the radionuclide source. The per cent deviation ranged from 0.8% for 18F to a maximum of −11.6% for a 68Ga radionuclide source. Uncertainties of this magnitude are well below the accepted uncertainties of commonly used survey meters in PET departments, which are of the order of ±30%.
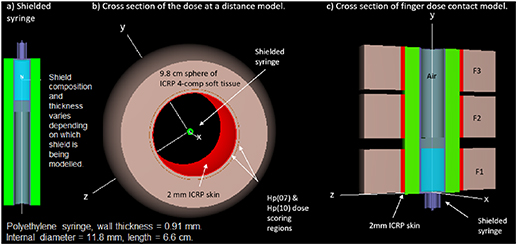
Figure 1. (a) Cross section of the soft tissue model from Amato et al [11] reproduced in EGSnrc. (b) Cross section of finger dose model from Italiano et al [12] reproduced in EGSnrc.
Download figure:
Standard image High-resolution imageAs a further validation of EGSnrc, we reproduced the geometries used by Italiano et al [12] for modelling the fingers of an operator in direct contact with a receptacle containing 18F. The GEANT-4 simulations by Italiano [12] considered a cylindrical syringe composed of polyethylene (σ = 0.94 g cm−3), with wall thickness of 1 mm, inner diameter of 1.0 cm and length of 4 cm. 18F was homogeneously distributed in a water solution, which filled the syringe volume up to a length of 2 cm and the remainder of the syringe contained air. Italiano et al [12] simulated three different finger locations in contact with the syringe wall (figure 1(b)). The fingers were represented as three individual annuli of soft tissue (ICRP4 component soft tissue σ = 1.0 g cm−3), with 1 cm thickness. Hp(0.07) was scored at a depth of 65–75 µm. All geometries were reproduced in EGSnrc, although there was a small difference in the density of polyethylene used (0.93 g cm−3 in EGSnrc compared to σ = 0.94 g cm−3 in GEANT4). The modelled doses using EGSnrc were within −6% and 2% of those reported using GEANT-4 for each simulated finger position for 18F.
2.1.1. Syringe source model.
After the validation procedure was completed, the syringe model was optimised to more accurately reflect the actual syringe used in our institution, namely the BD Plastipak 5 ml syringe [17]. This cylindrical syringe has a uniform polypropylene (ρ = 0.9 g cm−3) wall of 0.091 cm thickness and an internal diameter of 1.18 cm (figure 2(a)). In our model, the needle end of the syringe replicated the Luer-Lok. The radioactive water solution was contained within the 1st 2–2.2 ml of the syringe followed by a 0.5 cm thick cylindrical disc of polypropylene, representing the cap of the plunger. The remainder of the hollow syringe was filled with dry air. The plunger handle and needle were not included in this model.
Figure 2. (a) Cross section of shielded syringe. (b) Shielded syringe in a clipped spherical tissue shell. In the case of the eye dose model the spherical shell is composed entirely of ICRP soft tissue. (c) Cross section of the contact dose model for a shielded syringe, where F1, F2 and F3 are the simulated finger positions.
Download figure:
Standard image High-resolution image2.1.2. Syringe shield model.
The details of a selection of commercially available PET syringe shields modelled in this study are summarized in table 2.
Table 2. Commercially available syringe shields assessed in this study.
| Manufacturer | Model | Thickness (mm) | Composition | Density (g cm−3) | Attenuation of 511 keV |
|---|---|---|---|---|---|
| Britec | PET syringe shield | 7.5 [18] | 90% tungsten, nickle and copper mixed. | 18.3* | Not specified |
| Biodex | Pro-tec syringe shield | 9 [19, 20] | Tungsten | 19.3 | 88% |
| Biodex | Z-PET | 14 [21] | Tungsten | 19.3 | 97% |
| Britec | Lead glass | 11–13 [18] | 4 mm lead equivalent | 11.4 | Not specified |
Note:*The density of the 7.5 mm Britec shield syringe was calculated by assuming a composition of 90% W, 5% Cu and 5% Ni.
The thickness and composition of the shield for each simulation was modified to reflect the actual shield being assessed. While EGSnrc contained tungsten and lead density correction files, it was necessary to create a unique density correction file for the Britec tungsten composite 7.5 mm syringe shield using energy response data from NIST [22].
2.1.3. Soft tissue model.
The spherical soft tissue phantom employed by Amato et al [11] simulates the average exposure at any orientation at the defined distance from the syringe; this is a reasonable approach for uniform isotropic sources. However, in practice the syringe is not shielded entirely as the syringe is tubular and is open at both ends. When the tubular syringe shield is in place positrons and photons emitted from the unshielded ends of the syringe can also contribute to exposure. In order to exclude the direct irradiation from the unshielded portion of the syringe the spherical phantom was clipped by two bounding planes, optimally positioned for each source to detector distance. The dose rates simulated using this model provide a reasonable estimation of the average dose a person would receive at a distance from the shielded barrel of the syringe. However, a higher dose would be received if a person was positioned directly in front of or behind the shielded syringe. In that situation the dose would be significantly higher. A further modification was made to the soft tissue shell to replace the 1st 2 mm of layer of soft tissue with 2 mm skin (ICRP skin σ = 1.1 g cm−3) for assessment of Hp(0.07) and Hp(10) (figure 2(b)). For simulation of Hp(3) the spherical shell was composed entirely of ICRP4 component soft tissue with no skin layer. The eye dose scoring volume was defined as a spherical shell at the tissue equivalent depth of the sensitive volume of the eye lens, between 2.80 mm and 3.82 mm contained inside the absorber [23].
2.1.4. Contact dose model.
The contact finger dose model implemented in this study was based on the model by Italiano et al [12]. Three different finger locations were simulated in contact with the syringe wall. The finger model employed in this study consisted of 2 mm skin in contact with the syringe, followed by 1.8 cm of soft tissue (ICRP4 component soft tissue σ = 1.0 g cm−3) for a total thickness of 2 cm and a width of 2 cm. Hp(0.07) was scored at a depth of 65–75 µm. The contact dose model employed in our study considered only one finger (F1) to have direct contact with the portion of the syringe containing the radioactive solution (figure 2(c)). This position corresponds to the maximum dose equivalent to which an operator can be exposed to when manipulating the syringe. The remaining two fingers (F2 and F3; figure 2(c)) were positioned over the air-filled section of the syringe. The syringe modelled for contact dose was considered to have a total length of approximately 6.7 cm to accommodate the three finger positions and the radioisotope was homogeneously distributed in a water solution up to a height of 2 cm (volume 2.2 ml). A cross section of the syringe simulation models used in this study are illustrated in figure 2.
2.2. Experimental validation of modelled doses
Dose rate measurements were obtained for the two isotopes available in our centre, namely 18F and 68Ga, for a shielded and unshielded syringe. The syringe shield used in our centre is the 7.5 mm Britec PET Shield [18]. Hp(10) was measured using the Radeye G20-ER survey meter [24] at a distance of 30 cm from the shielded and unshielded syringe. Hp(0.07) was assessed using the Radeye B20-ER survey meter [25] for the same geometry. The Radeye B20-ER was used without a filter which yields the measurement of the directional dose equivalent for beta radiation (100 keV–800 keV average energy). The results obtained are shown in table 3.
Table 3. Comparison between EGSnrc simulation and dose rate measurements at 30 cm from an unshielded and shielded syringe.
| Deep dose rate (µGy/MBr.hr) | Skin dose rate (µGy/MBr.hr) | |||||
|---|---|---|---|---|---|---|
| EGSnrc | Radeye G20-ER | % diff | EGSnrc | Radeye B20-ER | % diff | |
| Unshielded syringe | ||||||
| 18F | 1.81 ± 0.01 | 1.80 ± 0.02 | −0.6% | 2.6 ± 0.1 | 2.4 ± 0.02 | −8.3% |
| 68Ga | 1.93 ± 0.02 | 2.11 ± 0.30 | 8.5% | 24.8 ± 0.2 | 35.4 ± 0.84 | 29.9% |
| Shielded syringe | ||||||
| 18F | 0.49 ± 0.002 | 0.51 ± 0.01 | 3.9% | 0.90 ± 0.01 | 0.61 ± 0.01 | −47.5% |
| 68Ga | 0.51 ± 0.01 | 0.54 ± 0.01 | 5.6% | 2.27 ± 0.05 | 1.91 ± 0.21 | −18.8% |
The response of both survey meters, as quoted by the manufacturer and confirmed by annual calibration with an ISO-17025 accredited calibration service laboratory, yields doses with a measurement tolerance of ±30%. Deep dose rate measurements with the Radeye G20-ER for both isotopes compare very well with the EGSnrc simulated values, for the unshielded and shielded syringe model. The skin dose measurements were all within the tolerance of the Radeye B20-ER survey meter, with the exception of the shielded syringe for 18F. Survey meter measurements were performed with a syringe capped with a needle and plastic cover, and the stem of the plunger was also in place. This geometry differs slightly from the simulated syringe model, where both ends are capped with plastic discs. In addition, the EGSnrc simulations provide the average dose rate at any orientation at a set distance from the syringe, excluding directly in front of or behind the syringe. Measurements were performed in a single orientation, with the survey meter parallel to the syringe barrel. The closer a person is aligned to the edge of the syringe, the more exposed they are to the radiation escaping from the unshielded ends of the tubular syringe. Taking all this into consideration, the Radeye B20-ER measured skin dose rates for 68Ga and 18F were considered to compare reasonably well with the EGSnrc simulated values for both the unshielded and shielded syringe model.
3. Results
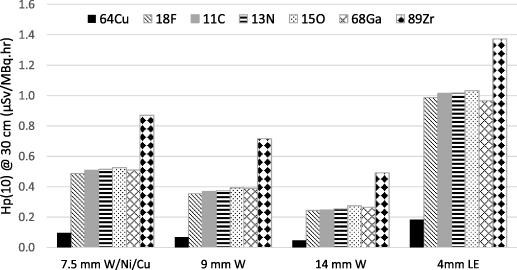
EGSnrc simulation results for dose rates at a distance of 30 cm from the unshielded and shielded syringes are presented here. The results for distances of 10 cm and 50 cm from the syringe are presented in tables S1 and S2, respectively in the supplementary data section (available online at stacks.iop.org/JRP/41/1060/mmedia). As 82Rb is prepared and dispensed through an automated infusion system, it is very unlikely to be stored or manipulated in a syringe. However, once this dispensing is complete, it is often administered to the patient through an unshielded administration line. The dose rates for 82Rb in an unshielded syringe, while not directly clinically relevant, are presented here to illustrate the potential risk of occupational exposure from unshielded sources of 82Rb. The results of EGSnrc simulations for Hp(0.07), Hp(3) and Hp(10) at a distance of 30 cm from the unshielded and shielded syringe are shown in table 4, and the skin dose rates Hp(0.07) for each shield are illustrated in figures 3 and 4, respectively.
Figure 3. Skin dose rates Hp(0.07) at 30 cm from the shielded syringes for each isotope.
Download figure:
Standard image High-resolution imageFigure 4. Deep dose rates Hp(10) at 30 cm from the shielded syringes for each isotope.
Download figure:
Standard image High-resolution imageTable 4. Dose rate as a distance of 30 cm from the unshielded and shielded syringe.
| Dose rate (µSv/MBq.hr) at 30 cm | ||||||||
|---|---|---|---|---|---|---|---|---|
| 64Cu | 18F | 11C | 13N | 15O | 68Ga | 89Zr | 82Rb | |
| Hp(0.07) | ||||||||
| No syringe shield | 0.53 | 2.61 | 6.17 | 10.68 | 22.98 | 24.80 | 3.66 | 54.69 |
| 7.5 mm W/Ni/Cu | 0.15 | 0.90 | 1.04 | 1.31 | 2.16 | 2.27 | 1.11 | — |
| 9 mm W | 0.13 | 0.65 | 0.89 | 1.11 | 1.99 | 2.11 | 0.95 | — |
| 14 mm W | 0.09 | 0.45 | 0.63 | 0.86 | 1.73 | 1.95 | 0.70 | — |
| 4 mm Pb (lead glass) | 0.36 | 1.90 | 2.14 | 2.37 | 3.22 | 3.21 | 1.81 | — |
| Hp(3) | ||||||||
| No syringe shield | 0.36 | 1.90 | 1.99 | 2.06 | 3.30 | 4.11 | 2.12 | 29.30 |
| 7.5 mm W/Ni/Cu | 0.10 | 0.52 | 0.54 | 0.54 | 0.61 | 0.62 | 0.90 | — |
| 9 mm W | 0.07 | 0.37 | 0.39 | 0.40 | 0.46 | 0.49 | 0.76 | — |
| 14 mm W | 0.05 | 0.26 | 0.27 | 0.28 | 0.33 | 0.37 | 0.54 | — |
| 4 mm Pb (lead glass) | 0.19 | 1.05 | 1.06 | 1.08 | 1.13 | 1.11 | 1.42 | — |
| Hp(10) | ||||||||
| No syringe shield | 0.34 | 1.81 | 1.87 | 1.92 | 2.06 | 1.93 | 1.94 | 3.17 |
| 7.5 mm W/Ni/Cu | 0.10 | 0.49 | 0.51 | 0.51 | 0.53 | 0.51 | 0.87 | — |
| 9 mm W | 0.07 | 0.36 | 0.37 | 0.37 | 0.39 | 0.39 | 0.72 | — |
| 14 mm W | 0.05 | 0.24 | 0.25 | 0.25 | 0.28 | 0.27 | 0.54 | — |
| 4 mm Pb (lead glass) | 0.18 | 0.99 | 1.02 | 1.02 | 1.03 | 0.97 | 1.37 | — |
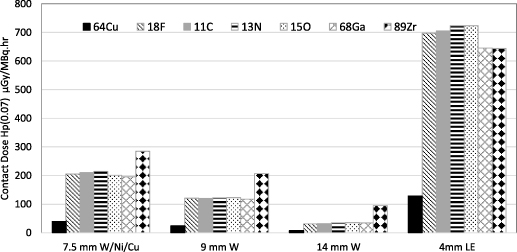
The results of EGSnrc simulations for contact skin exposure rates from the unshielded syringe are shown in table 5. The results of the shielded syringe simulations are illustrated in figure 5 and the corresponding data are provided in table S3 in the supplementary data section.
Figure 5. Contact skin dose rates Hp(0.07) for the shielded syringes at finger position F1.
Download figure:
Standard image High-resolution imageTable 5. Simulated contact skin dose rates for the unshielded syringe.
| Contact skin dose rate Hp(0.07) (mSv/MBq.hr) | |||
|---|---|---|---|
| Isotope | Finger position F1 | Finger position F2 | Finger position F3 |
| 64Cu | 0.73 | 0.05 | 0.01 |
| 18F | 3.08 | 0.30 | 0.07 |
| 11C | 8.48 | 0.30 | 0.07 |
| 13N | 14.83 | 0.31 | 0.07 |
| 15O | 32.12 | 0.32 | 0.08 |
| 68Ga | 34.27 | 0.33 | 0.07 |
| 89Zr | 4.54 | 0.36 | 0.09 |
| 82Rb | 75.13 | 1.65 | 0.19 |
Table 6 displays the conversion factors to estimate Hp(0.07) from Hp(10) and Hp(3) from Hp(10) at 30 cm from the syringe, for each isotope.
Table 6. Conversion factor to estimate Hp(0.07) and Hp(3) from Hp(10) at 30 cm from the syringe.
| Conversion factor to estimate Hp(0.07) from Hp(10) at 30 cm | ||||||||
|---|---|---|---|---|---|---|---|---|
| 64Cu | 18F | 11C | 13N | 15O | 68Ga | 89Zr | 82Rb | |
| No syringe shield | 1.6 | 1.4 | 3.3 | 5.6 | 11.2 | 12.8 | 1.9 | 17.2 |
| 7.5 mm W/Ni/Cu | 1.5 | 1.8 | 2.0 | 2.6 | 4.1 | 4.5 | 1.3 | — |
| 9 mm W | 1.9 | 1.9 | 2.4 | 3.0 | 5.1 | 5.4 | 1.3 | — |
| 14 mm W | 1.9 | 1.8 | 2.6 | 3.4 | 6.3 | 7.3 | 1.4 | — |
| 4 mm Pb (lead glass) | 2.0 | 1.9 | 2.1 | 2.3 | 3.1 | 3.3 | 1.3 | — |
| Conversion factor to estimate Hp(3) from Hp(10) at 30 cm | ||||||||
| No syringe shield | 1.06 | 1.05 | 1.07 | 1.07 | 1.60 | 2.13 | 1.09 | 9.23 |
| 7.5 mm W/Ni/Cu | 1.07 | 1.06 | 1.06 | 1.05 | 1.16 | 1.21 | 1.04 | — |
| 9 mm W | 1.12 | 1.05 | 1.06 | 1.06 | 1.19 | 1.26 | 1.06 | — |
| 14 mm W | 1.08 | 1.06 | 1.10 | 1.10 | 1.21 | 1.40 | 1.09 | — |
| 4 mm Pb (lead glass) | 1.05 | 1.07 | 1.04 | 1.07 | 1.09 | 1.15 | 1.04 | — |
The conversion factors to estimate Hp(3) from Hp(10) were found to be between 1.0 and 1.3 for all geometries for 64Cu, 18F, 11C, 13N and 89Zr. It is, thus, reasonable to estimate the eye dose as being approximately equal to the deep dose for these isotopes, for all geometries. For 15O, 68Ga and 82Rb in the case of the unshielded syringe, the deep dose to eye dose conversion factors were found to be approximately 1.6, 2.1 and 9.2, respectively. These conversion factors reduce to between 1.1 and 1.2 and 1.2 and 1.4 for 15O and 68Ga, respectively, when any of the syringe shield assessed in this study are in place. These conversion factors can be used to estimate the skin and eye dose rates in situations where the deep dose rate can be measured in real time or is known.
4. Discussion
Manipulation of unsealed positron-emitting radionuclides can result in significant skin doses to the hands of staff during preparation (labelling/dispensing) and administration of the PET radiopharmaceuticals. The occupational exposure will depend not only on the activity of the radionuclide but also on the radiation protection measures employed (time, distance, and shielding). PET centres that have exclusively used 18F should be aware of the potential for much higher occupational extremity exposure with the introduction of higher energy positron emitting nuclides, with dose rates from unshielded sources significantly higher than that of 18F. The contact skin dose rate for an unshielded syringe containing 68Ga was found to be 11 times higher than that for 18F, when the syringe is held close to the active volume (finger position F1). This is in excellent agreement with previously published values [6]. Based on the simulation results for F1 obtained in this study, the time taken to reach the annual dose limit of 500 mSv for an operator holding an unshielded syringe of 200 MBq would be approximately 205, 48, 18, 10, 5, 4, 33 and 2 min for 64Cu,18F, 11C, 13N, 15O, 68Ga, 89Zr and 82Rb, respectively. Thus, care must be taken to avoid direct or indirect exposure to unshielded radionuclide sources; syringe shields and forceps should be used where possible when handling unsealed radioactive sources.
As most commercially available PET syringe shields have a thickness of the order of 7–14 mm of tungsten, they should effectively absorb the positrons from all isotopes assessed in this study and significantly attenuate the gamma radiation. The addition of a syringe shield achieves a significant reduction in the contact skin dose for all isotopes. The 7.5 mm W/Ni/Cu shield achieved a reduction in Hp(0.07) of 96.7% ± 2.7% at finger position F1. The percentage reduction at position F1 for the 9 mm W shield was 98.3% ± 1.8%, and 99.4% ± 0.7% for the 14 mm W shield. The 4 mm lead equivalent shield, simulating the lead glass portion of the shield, achieved a reduction of rate of 91.9% ± 7.7%. The contact skin dose rate for 64Cu is significantly lower than that of the other isotopes; for all shields, it is approximately 20% of the contact skin dose rate of 18F. With the exception of the lead glass shield, the contact skin dose rate for 89Zr is notably higher than that of the other isotopes; it is approximately 1.4, 1.7 and 3.1 times the contact skin dose rate of 18F, for the 7.5 mm, 9 mm and 14 mm shield, respectively. The contact skin dose rate is approximately equal for all other isotopes for each shield type, as there is very little contribution in this geometry to the finger dose from the positrons at the unshielded ends of the syringe shield. In all cases the contact doses rates at positions F2 and F3 are significantly lower than that of F1. If the syringe must be handled directly, the operator should ensure that their fingers are not in contact with the shield at a position directly over the radionuclide source and should also avoid directly handling the lead glass viewing window portion of the syringe.
The syringe shields are specified by their attenuation of the 511 keV annihilation photons, which should correspond to the specified reduction in deep dose. For the realistic tubular syringe model simulated in this study, the attenuation of photons at a distance of 30 cm from the source, as measured by Hp(10), was found to be of the order of 73%, 80%, 87% and 47% for the 7.5 mm, 9 mm, 14 mm and 4 mm shields, respectively, for all isotopes excluding 89Zr. The specification is most likely based on the attenuation offered by the composition of the syringe material in a simple geometry. It does not take into account the high energy positrons and gamma photons escaping from the unshielded portion of the syringe, which contribute to an increase in Hp(0.07), Hp(3) and Hp(10) at a distance from the syringe. This effect is not evident when the operator's hands are in direct contact with the syringe.
The abundant presence of the highly penetrating 909 keV photons of 89Zr results in less effective attenuation for the shields assessed in this study for this isotope. The attenuation of 89Zr photons at a distance of 30 cm from the source, as measured by Hp(10), was found to be of the order of 55%, 63%, 75% and 29% for the 7.5 mm, 9 mm, 14 mm and 4 mm shields, respectively. This is notably less than for the other isotopes assessed in this study. While Hp(10) at a distance of 30 cm from the unshielded syringe containing 89Zr is approximately the same as that of 18F, when any of the shields assessed in this study are in place, Hp(10) for 89Zr is approximately twice that of 18F. Alzimami et al [26] compared the occupational exposure to staff as a result of emissions from patient administrations of 18F and 89Zr. They found that, in close proximity to the patient source, the dose rates where similar for both isotopes (within 20%). However, when the dose rate was measured outside the room, the dose rate from 89Zr was up to 22 times higher than that of 18F, owing to the reduced attenuation of the high energy 909 keV 89Zr photon by the structural shielding. Holland et al [27] observed that cyclotron operators and radiochemists working in facilities originally designed for 18F experience large increases in occupational doses when handling 89Zr. The results obtained here further confirm that special consideration should be given to radiation protection of staff when 89Zr is used in the department. 89Zr will require additional shielding for transport and safe handling than is generally considered adequate for pure positron emitters such as 18F.
For the remaining isotopes in this study, when any of the syringe shields are in place, Hp(10) simulated values at a distance of 30 cm from the shielded syringe, are comparable to 18F for all isotopes (within 14%); with the exception of 64Cu whose deep dose rate is only 20% of 18F. However, the Hp(0.07) estimate is in some cases, significantly higher than 18F for the isotopes studied, again with the exception of 64Cu.
Eye doses are not usually of concern for isotopes with a maximum positron energy below 700 keV [23], such as 18F and 64Cu. However, for the other PET isotopes considered in this study there is the potential for an increased eye dose, particularly in situations where the radionuclide source is not shielded. While the eye dose model presented here is a simplified model based on the tissue equivalent depth of the eye, it provides a useful estimation of the eye dose. Simulations in this study suggest that Hp(3) at a distance of 50 cm from an unshielded syringe are comparable to 18F for 11C, 13N and 89Zr (within 30%). In contrast, the eye doses for 15O, 68Ga, and 82Rb were found to be 1.6, 2 and 14 times that of 18F, respectively. Taking these Hp(3) estimations at 50 cm from an unshielded source of 200 MBq, the time taken to reach the annual limit of 20 mSv would be approximately 88 h, 73 h and 10 h for 15O, 68Ga and 82Rb, respectively. Eye dose monitoring may be required if high activities of unshielded sources of 68Ga, 15O or 82Rb are in use in the department. In the case of patient administration through an unshielded line, the distance between the line and the operator, shielding between the line and the operator, the workload and the infusion duration should also be taken into account.
When any of the syringe shields assessed in this study are in place, Hp(3) is comparable to that of 18F for all isotopes, with the exception of 89Zr. Due to the presence of the highly penetrating 909 keV gamma photon, Hp(3) for 89Zr is approximately twice that of 18F when a syringe shield is in place. 82Rb in a shielded syringe was not assessed as this case is not clinically relevant due to the automatic infusion method typically employed for 82Rb radiopharmaceuticals.
The results presented in this paper, both for the shielded and unshielded syringe, will apply whenever the operator is holding a 5 ml syringe or positioned at a distance from a 5 ml syringe. The syringe geometries and configurations simulated in this study are mostly relevant to dispensing and administration tasks performed by the operator. The preparation and QC tasks for some of the more novel radionuclides may require more complex manipulations in geometries not covered by the simulations in this study. Radionuclides with higher energy positrons and additional gamma photons compared to 18F, will also result in increased transmission rates if the radionuclide is in a smaller geometry syringe, for example in a 1 ml syringe for pre-administration QC, or other clinically relevant plastic configurations such as IV tubing.
The results of the simulations provided in this study can assist in the selection of the optimum syringe shield, although consideration is also needed for the workload and the associated administered activity of each isotope. Although the dose rates are higher for 68Ga than 18F, the administered activity and number of patients per week is generally much less for 68Ga than 18F. In our centre, the administered activity for 68Ga is 100–130 MBq, whereas the administered activity for 18F FDG is weight-based—3 MBq kg−1 up to a maximum of 300 MBq. Ease of use and weight should also be considered when selecting the appropriate syringe. As expected, the 14 mm tungsten shield provides the highest protection from the shields assessed in this study. However, as the thickness of the syringe shield increases, the weight also increases which can make the shield more challenging to use. The shields assessed in this study accommodate a 5 ml syringe. For this syringe size, the weight of the 14 mm shield is 1.7 kg compared to 0.77 kg for the 9 mm shield and 0.6 kg for the 7.5 mm shield. Another consideration is the degree of manipulation that is required by the operator. The heavier shields generally do not contain a lead glass viewing window. The presence of the viewing window can reduce the overall time the operator spends manipulating the source and can be useful when removing air-bubbles and confirming volumes. The dose rates from the lead glass viewing window are significantly higher than from the tungsten barrel of the syringe, so care must be taken to avoid direct contact with this portion of the shield. The attenuation provided by the shields assessed in this study may not be sufficient for use with 89Zr in the case of high activities being handled or high patient numbers. Special consideration should be given to the shielding used for the manipulation, storage, transport and administration of 89Zr, including the use of an automated delivery system.
5. Conclusion
Our study has shown that it is possible to make realistic estimates of Hp(10), Hp(0.07) and Hp(3) for a range of PET radionuclides and syringe shields using Monte Carlo simulation. The results of these simulations can be used to estimate conversion factors to determine Hp(0.07) and Hp(3) doses using real-time measurements of Hp(10). For the isotopes analysed in this study, with the exception of 64Cu, the positrons have a higher energy and range than those of 18F and can potentially result in significant skin dose for unshielded sources. Procedures which involve exposure to unshielded sources of high energy positron emitting isotopes should be assessed to ensure all appropriate radiation safety measures are employed to reduce staff doses. The appropriate shield for use with each isotope will depend on its ease of use and the range of isotopes and activities that will be handled. Special consideration should be given to the selection of appropriate shielding for 89Zr. Eye dose monitoring may be required if high activities of unshielded sources of 68Ga, 15O or 82Rb are in use in the department.
Acknowledgments
This work was funded by a Government of Ireland Postgraduate Scholarship from the Irish Research Council (GOIPG/2019/3652).