Abstract
In this work, we have investigated the crystal and electronic structure of the orthorhombic phase of BaPbxBi O3 (BPBO) for x = 0.7 (BPBO70), 0.75 (BPBO75) and 1.0 (BPO), using temperature dependent x-ray diffraction measurements, photoemission spectroscopy, and electronic structure calculations. Our results show the importance of particle size and strain in governing superconductivity. Interestingly, the temperature evolution of the structural parameters in the case of BPBO70 is similar to that of BPBO75 but the magnitude of the change is diminished. The BPBO75 and BPO compounds exhibit metallic nature, which is corroborated by the core level studies. The electronic structure calculations in conjunction with the core level studies suggest that oxygen vacancies play an important role for metallicity observed in the end compound. The exponent to the spectral line shape close to the Fermi level suggests the origin of pseudogap to be due to other contributions in addition to disorder in the case of BPBO70 and BPO. The core level studies also show that as one goes from x = 0.70 to 1.0, there occurs chemical potential shift towards the valence band suggesting hole doping. Our results open the venue to further study these compounds as a function of particle size, nature of carriers for its transport behaviour, electronic structure below TC, composition at the grain boundaries and microscopic origin of pseudogap in the non-superconducting phase. We believe that our results call for a revision of the temperature-doping phase diagram of BPBO to include the pseudogap phase.
O3 (BPBO) for x = 0.7 (BPBO70), 0.75 (BPBO75) and 1.0 (BPO), using temperature dependent x-ray diffraction measurements, photoemission spectroscopy, and electronic structure calculations. Our results show the importance of particle size and strain in governing superconductivity. Interestingly, the temperature evolution of the structural parameters in the case of BPBO70 is similar to that of BPBO75 but the magnitude of the change is diminished. The BPBO75 and BPO compounds exhibit metallic nature, which is corroborated by the core level studies. The electronic structure calculations in conjunction with the core level studies suggest that oxygen vacancies play an important role for metallicity observed in the end compound. The exponent to the spectral line shape close to the Fermi level suggests the origin of pseudogap to be due to other contributions in addition to disorder in the case of BPBO70 and BPO. The core level studies also show that as one goes from x = 0.70 to 1.0, there occurs chemical potential shift towards the valence band suggesting hole doping. Our results open the venue to further study these compounds as a function of particle size, nature of carriers for its transport behaviour, electronic structure below TC, composition at the grain boundaries and microscopic origin of pseudogap in the non-superconducting phase. We believe that our results call for a revision of the temperature-doping phase diagram of BPBO to include the pseudogap phase.
Export citation and abstract BibTeX RIS
1. Introduction
From classical physics, we know that everything in motion undergoes energy loss in a dissipative medium. Dissipation in terms of heat has been utilised in our day today life for room heaters, light bulbs, cooking, hair dryers etc. Observation of flow of current without any dissipation is one of the bizarre effects that is counter intuitive that is realised in superconductors. Superconductivity is one such property where quantum effects are manifested at the macroscopic level. To understand the phenomena of superconductivity, it is important to understand the pre-requisite that leads to this phenomena. This means that the understanding of the state from which superconductivity arises is vital.
BaPbx
Bi O3 (BPBO), a bismuthate based superconductor with perovskite structure displays superconductivity in the composition range,
O3 (BPBO), a bismuthate based superconductor with perovskite structure displays superconductivity in the composition range,  [1, 2]. The parent compound, BaBiO3, stabilises in monoclinic structure and is semiconducting. In the monoclinic structure there occurs breathing mode distortion in the BiO6 octahedra that has been attributed to bond disproportionation [3–5]. The compounds in the superconducting compositional range—BPBO70 and BPBO75 – are known to show structural dimorphism; the coexistence of tetragonal I4/mcm and orthorhombic Ibmm phases [2, 6–8], with the 75% compound showing the highest superconducting transition temperature of
[1, 2]. The parent compound, BaBiO3, stabilises in monoclinic structure and is semiconducting. In the monoclinic structure there occurs breathing mode distortion in the BiO6 octahedra that has been attributed to bond disproportionation [3–5]. The compounds in the superconducting compositional range—BPBO70 and BPBO75 – are known to show structural dimorphism; the coexistence of tetragonal I4/mcm and orthorhombic Ibmm phases [2, 6–8], with the 75% compound showing the highest superconducting transition temperature of  K in the series [1]. The end compound, BPO, stabilises in orthorhombic Ibmm space group and is metallic [9, 10]. From structural point of view, it has been observed that in the compositional regime that stabilises superconductivity has orthorhombic and tetragonal structure. It has also been found that the tetragonal structure helps in the stabilisation of superconductivity [8]. Nanoscale structural phase separation was observed in the investigation of BPBO by Giraldo-Gallo et al using high quality single crystals [7]; the structural dimorphism was observed to take the form of partially disordered stripes, the width of which was comparable to Ginzburg–Landau coherence length at optimal composition (x ≈ 0.24).
K in the series [1]. The end compound, BPO, stabilises in orthorhombic Ibmm space group and is metallic [9, 10]. From structural point of view, it has been observed that in the compositional regime that stabilises superconductivity has orthorhombic and tetragonal structure. It has also been found that the tetragonal structure helps in the stabilisation of superconductivity [8]. Nanoscale structural phase separation was observed in the investigation of BPBO by Giraldo-Gallo et al using high quality single crystals [7]; the structural dimorphism was observed to take the form of partially disordered stripes, the width of which was comparable to Ginzburg–Landau coherence length at optimal composition (x ≈ 0.24).
Several photoemission studies have been reported on BPBO, so far [11–14]. However, most of these studies are at room temperature or liquid nitrogen temperature. Detailed temperature evolution studies have not yet been reported. Also, it is important to note that for the stabilisation of the superconducting phase, sample preparation condition plays an important role. In literature, studies comparing the superconducting and the non-superconducting properties with preparation conditions is lacking. Such studies will help in understanding the precursors to superconductivity in the compound.
The compositional region in which BPBO70 and BPBO75 lie has been identified to be the region of metal to semiconductor transition [15], which implies the coexistence of metallic and semiconducting regions. Thus, these compositions are expected to show a pseudogap. In the underdoped region of cuprate superconductors the pseudogap phase is also detected, which has been interpreted in terms of preformed pairs, a circulating current phase, or exotic fractionalised states with topological order [16]. This pseudogap generally evolves into superconductivity below TC
. Interestingly, the existence of pseudogap has been observed in BPBO system in the compositional region  by Nagoshi et al [17] based on their room temperature (RT) valence band studies. However, there are no further studies on the evolution of this pseudogap with temperature or composition. It is interesting to note that they did not observe metallicity or finite density of states (DOS) in the end compound, despite previous reports [9, 10, 18] indicating that the sample is metallic. This could be due to the poor base pressure
by Nagoshi et al [17] based on their room temperature (RT) valence band studies. However, there are no further studies on the evolution of this pseudogap with temperature or composition. It is interesting to note that they did not observe metallicity or finite density of states (DOS) in the end compound, despite previous reports [9, 10, 18] indicating that the sample is metallic. This could be due to the poor base pressure  mbar and the feature due to the bands of carbonate and hydroxide occurring at 5 eV in the valence band spectrum which would lead to the intensity at the fermi level, EF
, to be underestimated.
mbar and the feature due to the bands of carbonate and hydroxide occurring at 5 eV in the valence band spectrum which would lead to the intensity at the fermi level, EF
, to be underestimated.
The 4+ valence state of Pb in BaPbO3 (BPO) results in an empty conduction band, and thus is expected to be semi-conducting. On the contrary, BPO is a metallic compound with electrons being the majority carriers [9, 10]. Band structure calculations identify BPO as a semi-metal [19, 20]. Ikushima and Hayakawa [9], on the basis of their electrical conductivity, hall mobility and electron-spin resonance (ESR) studies, propose that BPO is an oxygen deficient semiconductor with metallic conductivity whose conduction electrons reside in an impurity band consisting of excess donors. Shannon and Bierstedt [10] on the other hand, argue that high pressure and high temperature sintering of the BPO crystals in oxygen would result in the reduction of oxygen vacancies, and thus an increase in the resistivity. However such a change was not observed upon treatment at these extreme pressures, the resistivity of BPO remained unchanged. Yet another explanation put forth by Hsieh and Fu [18] is the presence of Pb2+ due to partial substitution of Ba site by Pb, resulting in a half-filled p-orbital, resulting in the observed metallic behaviour; an evidence supporting this claim was not observed in the studies by Medicherla et al [21]—no signatures of Pb2+ was found in the Pb 4f core level studies.
Some relevant questions that shape the understanding of the phase diagram of disordered superconductors are effects of doping and structural phase separation. In the present study, we present the detailed investigations of the crystal structure of the orthorhombic phase of BPBO (BPBO70, BPBO75 and BPO) and its effect on the electronic structure. We have performed temperature dependent x-ray diffraction (XRD) studies to elucidate the crystal structure of three compounds and its evolution with temperature. Our results show that in addition to lattice strain, particle size plays a significant role in the stabilisation of structural phase separation and hence superconductivity. The temperature dependent resistivity and magnetisation studies indicate that out of these compounds, only BPBO75 is superconducting. Our photoemission results show that for the lowest collected temperatures, all the compounds show pseudogap, with differing origins. PES studies along with DFT calculations suggest that the metallicity of the end compound is due to oxygen vacancies. For the first time, to the best of our knowledge, we are reporting the presence of pseudogap in BPO.
2. Experimental
2.1. Sample preparation
The powder samples of all compositions were prepared via the solid state route from BaCO3, Bi2O3 and Pb3O4. The raw materials were first preheated at 450  for 2 h to remove any traces of moisture absorbed by the raw materials. For the preparation of BPBO70 and BPO samples, proper stoichiometric ratios of the raw materials were ground thoroughly in an agate mortar for 6–10 h. This finely ground powder was then calcined in a box furnace at 900
for 2 h to remove any traces of moisture absorbed by the raw materials. For the preparation of BPBO70 and BPO samples, proper stoichiometric ratios of the raw materials were ground thoroughly in an agate mortar for 6–10 h. This finely ground powder was then calcined in a box furnace at 900  for 48 h. The heating rate was maintained at 5
for 48 h. The heating rate was maintained at 5  min−1 and the samples were allowed to cool down to room temperature naturally inside the box furnace. The calcined powders were then sintered in the box furnace at 900
min−1 and the samples were allowed to cool down to room temperature naturally inside the box furnace. The calcined powders were then sintered in the box furnace at 900  for around 48–96 h, with intermediate grinding every 24 h. In the case of BPBO75 sample, a similar procedure was adopted, the details of which are published elsewhere [22]. After each grinding, the powders were then pressed into pellets of 10 mm diameter under a hydrostatic pressure of 5 tons. It was found that covering the pellets with a thin layer of powder of the sample ensured a good stoichiometric compound, and prevented the escape of Bi or Pb from the surface of the pellet. It is to be noted that despite not sintering the sample in oxygen atmosphere, we were able to obtain a superconducting transition in the BPBO75 compound. The final sintering was at 950
for around 48–96 h, with intermediate grinding every 24 h. In the case of BPBO75 sample, a similar procedure was adopted, the details of which are published elsewhere [22]. After each grinding, the powders were then pressed into pellets of 10 mm diameter under a hydrostatic pressure of 5 tons. It was found that covering the pellets with a thin layer of powder of the sample ensured a good stoichiometric compound, and prevented the escape of Bi or Pb from the surface of the pellet. It is to be noted that despite not sintering the sample in oxygen atmosphere, we were able to obtain a superconducting transition in the BPBO75 compound. The final sintering was at 950  for 24 h. The BPBO70 sample was allowed to cool down to room temperature normally, where as, the BPO sample was cooled to room temperature over a period of 24 h. We observe a difference in both physical and structural properties due to the differences in the sample preparation routes.
for 24 h. The BPBO70 sample was allowed to cool down to room temperature normally, where as, the BPO sample was cooled to room temperature over a period of 24 h. We observe a difference in both physical and structural properties due to the differences in the sample preparation routes.
2.2. Characterisation
The temperature dependent XRD (T-XRD) measurements were performed using Rigaku's Smart Lab x-ray diffractometer powered by a 9 kW rotating anode x-ray generator. The T-XRD patterns were collected using Cu K radiations and were collected in the 2θ range of 19∘–87∘ at a scanning speed of 1∘ per minute with a step size of 0.02∘. The Magnetic Properties Measurement System from Quantum Design, Inc. was used in the measurement of temperature dependent DC magnetisation measurements. The measurement was performed at an applied magnetic field of 0.5 T, in the temperature range of 300 K to 2 K. Electrical resistivity measurements were performed on the sample using Physical Properties Measurement System setup from Quantum Design, Inc.
radiations and were collected in the 2θ range of 19∘–87∘ at a scanning speed of 1∘ per minute with a step size of 0.02∘. The Magnetic Properties Measurement System from Quantum Design, Inc. was used in the measurement of temperature dependent DC magnetisation measurements. The measurement was performed at an applied magnetic field of 0.5 T, in the temperature range of 300 K to 2 K. Electrical resistivity measurements were performed on the sample using Physical Properties Measurement System setup from Quantum Design, Inc.
The photoemission spectra were collected on Scienta R4000 hemispherical analyser using a monochromatic Al K (1486.6 eV) x-ray, He
(1486.6 eV) x-ray, He (40.8 eV) and He
(40.8 eV) and He (21. eV) sources. The binding energy scale was calibrated by measuring the Fermi level of Ag pellet, cleaned in-situ by scraping, using monochromatic Al K
(21. eV) sources. The binding energy scale was calibrated by measuring the Fermi level of Ag pellet, cleaned in-situ by scraping, using monochromatic Al K and He
and He . In-situ cleaning of the surface of a polycrystalline is commonly done using (a) scraping, (b) ion-sputtering and (c) fracturing. However, the work by Nagoshi et al [17, 23–25] show that scraping and ion-sputtering can lead to significant modifications of the surface states and that the best way of surface cleaning is by fracturing the sample in ultra-high vacuum. Keeping this in mind, a clean sample surface was obtained by fracturing the mounted samples in UHV. All the spectra were collected within 60 min from the time of fracturing and it was repeated for the next set of measurements to ensure a clean surface. The base pressure during the measurement was
. In-situ cleaning of the surface of a polycrystalline is commonly done using (a) scraping, (b) ion-sputtering and (c) fracturing. However, the work by Nagoshi et al [17, 23–25] show that scraping and ion-sputtering can lead to significant modifications of the surface states and that the best way of surface cleaning is by fracturing the sample in ultra-high vacuum. Keeping this in mind, a clean sample surface was obtained by fracturing the mounted samples in UHV. All the spectra were collected within 60 min from the time of fracturing and it was repeated for the next set of measurements to ensure a clean surface. The base pressure during the measurement was  mbar.
mbar.
3. Computational
Electronic structure calculations using self-consistent full potential linear augmented-plane-wave (LAPW) were performed using the code implemented in Elk [26]. We have used local density approximation (LDA) [27] for the exchange potential and Tran–Blaha modified Becke–Johnson (TB-mBJ) potential [28] for the correlation part. The calculations were performed using the room temperature structural parameters obtained from the refinement of the XRD patterns of Ibmm phase for BPBO70, and BPO compounds. In the case of BPBO75, two calculations were performed, one with the structural parameters of I4/mcm and the other using the structural parameters of the Ibmm phase; the DOS thus obtained were then added in the ratio of 70:30. In the unit cell constructed from the structural refinement of the compounds (which consists of four formula units), one atom of Pb was replaced by Bi, to account for the 70%/75% doping of Pb at the Bi-site in case of BPBO70 and BPBO75 compounds. The muffin-tin approximation for the potential well in the crystal lattice employed in the calculations used radii of 2.8, 2.56, 2.43 and 1.43 bohr for Ba, Bi, Pb and O, respectively. The difference in total energy required for the termination of self-consistent cycles was set to be less than 10−4 hartree/cell.
4. Results and discussions
4.1. General characterisation
The top row of figure 1 shows the field emission scanning electron (FE-SEM) micrographs of the BPBO70, BPBO75 and BPO pellets, respectively. The average grain sizes of the three compounds are listed in table 1. The BPBO70 and BPBO75 compounds which are expected to show similar physical and chemical properties, show a deviation in the grain size—the BPBO75 compound which was ground for a longer time has a much smaller grain size. Conversely, the BPBO70 sample has a much larger grain size. The BPO compound which was cooled slower than the others, shows the largest grain size.
Figure 1. The top panel shows the SEM micrographs of BPBO70, BPBO75, and BPO. Rietveld refinement of the XRD patterns of BPBO (a) BPBO70, (b) BPBO75, and (c) BPO collected at 10 K. The panels (d)–(f) show the peaks in the range 41∘–43∘ for the BPBO70, BPBO75 and BPO compounds, respectively. The panels (g)–(i) show the most intense peaks of the three compounds. '*' corresponds to reflections due to Cu K .
.
Download figure:
Standard image High-resolution imageTable 1. Grain sizes of the compound under study, calculated from the SEM micrographs.
| Compound |

|

|

|
|---|---|---|---|
| Grain size (µm) | 29.34 | 8.04 | 39.56 |
At RT, the parent compound BaBiO3 stabilises in the monoclinic structure in the I2/m space group. In this structure there is both breathing and tilting mode distortions. This breathing mode distortion is driven by the bond disproportionation of the Bi–O bond:
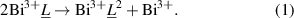
As we begin to introduce Pb into the compound, at Bi site, there is random occupation of Pb4+ ions in the Bi–O6 octahedra. Since the size of the Pb4+ is smaller than the Bi3+ ions, the average size of the Bi3+–O6 octahedra are expected to reduce. This will reduce the size difference between the Bi3+–O6 and the Bi –O6 octahedra, thus reducing the breathing mode distortion. Furthermore, upon Pb doping of the Bi-sites in BBO, the average ionic radii of the B-site cations reduces, resulting in the improvement of the Goldschmidt tolerance factor (equation (2)),
–O6 octahedra, thus reducing the breathing mode distortion. Furthermore, upon Pb doping of the Bi-sites in BBO, the average ionic radii of the B-site cations reduces, resulting in the improvement of the Goldschmidt tolerance factor (equation (2)),
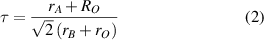
leading to the stabilisation of the compounds in the higher symmetry orthorhombic and/or tetragonal phases, above x = 0.2. As a result of this structural transformation, the number of peaks in the Pb-doped compound reduces. This is especially visible in the higher 2θ ranges, as seen from the insets (b1) and (b2) of figure 1(b).
In figures 1(a)–(c), we show the typical Rietveld fitting of the XRD patterns of BPBO collected at 10 K. The BPBO70 compound was refined using orthorhombic structure with space group Ibmm. We were able to index all the peaks with a single Ibmm phase, indicating that the compound is single phase. On the other hand, a two phase refinement was carried out for the BPBO75 compound using I4/mcm and Ibmm phases, whose crystal structure properties have been reported in our previous work [22]. The BPO compound stabilises in Ibmm space group of the orthorhombic crystal structure. The room temperature crystal structure and the lattice parameters of BPBO75 and BPO are in good agreement with the values reported in literature [6, 21]. Panels (d)–(f) of figure 1 show the (004) and (220) peaks in the three compounds.
It is worth noting that various studies [2, 6, 7] have reported that the BPBO70 compound is diphasic. Chemically, the two compounds BPBO70 and BPBO75, are very similar, and are hence expected to show similar structure. However, in the present study, we observe that the BPBO70 compound does not show phase separation (detectable by conventional XRD), where as the BPBO75 is diphasic. The difference in the two compounds arises due to the change in the sample preparation conditions.
In the orthorhombic crystal structure, unlike the monoclinic phase, there is only one crystallographic position for the B-site atom (Bi/Pb), which effectively quenches the breathing mode distortions. This is also very clearly visible in the Raman spectra of BPBO, where the 569 cm−1 peak which is associated with the breathing mode of BaBiO3, is strongly suppressed above a Pb concentration of 20% [29].
BaBiO3 exhibits an anisotropy in the Bi-O bonds along the apical and the basal planes. The Bi–O1 (apical) bonds are slightly smaller than the Bi–O2 (basal) bonds in the larger octahedra and the trend reverses in the smaller octahedra. As the Pb concentration increases, we see the anisotropy increase as well, with the orthorhombic phase of BPBO75 having the largest anisotropy between the apical and the basal bond lengths ( ). This anisotropy nearly vanishes in case of BPO, which has nearly identical apical and basal bonds lengths (
). This anisotropy nearly vanishes in case of BPO, which has nearly identical apical and basal bonds lengths ( ). This reduction in the Bi–O bond length anisotropy is visible in the reduced orthorhombicity of the end compound (as discussed on the forth-coming section).
). This reduction in the Bi–O bond length anisotropy is visible in the reduced orthorhombicity of the end compound (as discussed on the forth-coming section).
The top panel of figure 2 shows the magnetic moment of the BaPb0.7Bi0.3O3 (BPBO70) and BaPb0.75Bi0.25O3 (BPBO75) as a function of temperature which is consistent with the previous reports [6]. Both the compounds are diamagnetic across the whole temperature range; these samples show sharp drop at ∼10 K and ∼11 K, respectively, indicative of the superconducting transition. In the right panel of figure 2, we have shown the resistivity versus temperature data for the BPBO70 compound. It is evident from the graph that it shows semiconductor-like behaviour. The BPBO75 compound, on the other hand, shows a sharp transition at ∼11 K, indicating a superconducting transition, the details of which are reported elsewhere [22].
Figure 2. Left: Variation of magnetic moment with temperature of BPBO (BPBO70 and BPBO75). The inset shows its closer view in the temperature range 2 K–27 K. Right: Variation of resistivity with temperature of BPBO (BPBO70 and BPBO75).
Download figure:
Standard image High-resolution imageBPBO70 is reported [6] to be di-phasic with the coexistence of both orthorhombic (Ibmm) and tetragonal (I4/mcm) phases at all temperatures below 150  . The Rietveld refinements of our XRD data revealed that the compound has crystallised in a single phase—orthorhombic Ibmm space group. This however is not very uncommon, as there have been reports of finding monophasic BPBO70 [30–34]. However, unlike the previous reports, we observe a semiconductor-like behaviour of the dc resistivity, even though, a superconducting-like transition was observed in the dc susceptibility measurements at around 10 K. Revisiting the SEM micrographs shown in figure 1 gives us a clue regarding the absence of superconducting transition in the resistivity data. It is quite easily visible that the sample density of BPBO70 compound is lower compared to the BPBO75 sample. A similar observation has been made by Marx et al [6]. Also, it is worth noting that the size of the grains is much larger in case of BPBO70, as compared to BPBO75.
. The Rietveld refinements of our XRD data revealed that the compound has crystallised in a single phase—orthorhombic Ibmm space group. This however is not very uncommon, as there have been reports of finding monophasic BPBO70 [30–34]. However, unlike the previous reports, we observe a semiconductor-like behaviour of the dc resistivity, even though, a superconducting-like transition was observed in the dc susceptibility measurements at around 10 K. Revisiting the SEM micrographs shown in figure 1 gives us a clue regarding the absence of superconducting transition in the resistivity data. It is quite easily visible that the sample density of BPBO70 compound is lower compared to the BPBO75 sample. A similar observation has been made by Marx et al [6]. Also, it is worth noting that the size of the grains is much larger in case of BPBO70, as compared to BPBO75.
Usually, it is expected that with an increase in grain size, the number of grain boundaries would reduce leading to increased inter-grain connectivity. It is interesting to note that in the compounds under study, that shows superconductivity (BPBO75) has a lower grain size as compared to the non-superconducting phase, BPBO70. For practical applications of superconductors, it is required not only to have high transition temperature (TC ) but also high critical current density JC , i.e. the ability to carry large currents without resistance. With regard to the tuning of JC , the microstructure details of the sample play an important role. These results also emphasise the need for investigation of the concentrations of oxygen and Bi/Pb ions at the grain boundaries.
4.2. Temperature dependent XRD measurements
To understand the structural link with the transport properties, we have carried out temperature dependent XRD on all three compounds. Various lattice parameters, bond lengths and angles were obtained from the Rietveld refinement of the collected XRD patterns. The behaviour of the lattice parameters are illustrated as a function of temperature in figure 3.
Figure 3. Variation of lattice parameters with temperature for BPBO70 (open squares), BPBO75 (open circles), and BPO (open rhombus). Panels (a)–(c) show the variation of lattice parameters a, b, and c, respectively. Panels (d)–(f) show the temperature variation of the lattice parameters normalised with respect to their respective values at RT. Dashed lines are a visual guide indicating the overall behaviour of the various parameters.
Download figure:
Standard image High-resolution imageThe variations of the various lattice parameters can be divided into four regions for the BPBO70, BPBO75 compounds, based on the change in the slope. This change in slope is very evident in the variation of the lattice parameters, a, b and c, normalised with respect to their value at room temperature. In the temperature range 300 K–220 K, all three lattice parameters a, b and c of BPBO75, are found to reduce monotonically. As the temperature drops, for T < 220 K the lattice parameters a and b show a broad minimum and maximum around 125 K respectively; for T < 50 K, there occurs a cross-over in their behaviour. The c parameter is found to decrease until 25 K. Below 25 K, all three lattice parameters become constant. A similar trend is observed in case of BPBO70 compound as well, however, the variations are much smaller. The similarity between behaviour of the lattice parameters of the two compounds indicates that the variations of the lattice parameters of the orthorhombic phase of BPBO75 appear enhanced due to the presence of the tetragonal phase. In case of the end compound, the lattice parameter a shows a muted response to variations in temperature—a slight reduction is observed until 200 K, beyond which it increases monotonically until 10 K. On the other hand, b and c show a steady decrease until 25 K, beyond which we observe a slight increase.
Figures 4(a)–(c) shows the variation of the strain as a function of temperature in the three planes that bound the unit cell—ab, ac and bc planes. In case of BPBO75 compound, the orthorhombic strain becomes a maximum as the temperature nears the TC in the ab plane, as noted previously [22]. On the other hand, in the ac and bc planes, the strain reduces with temperature, attaining a minimum near TC . In case of the non-superconducting BPBO70 compound, the strain in the ab plane is nearly constant, and the strain in the bc and ac planes reduces monotonically with decreasing temperature and attains a minimum when approaching the expected superconducting transition temperature. In case of BPO compound, the orthorhombic strain steadily increases in the ab plane with decreasing temperature, attaining a maximum around 25 K, and reducing slightly below 25 K. The strain in the ac plane, on the other hand, reduces until ∼10 K; the strain in the bc plane remains almost unchanged with temperature. When we compare the BPBO70 and BPBO75 compounds, where the former is non-superconducting and the latter is superconducting, the orthorhombic strain is found to increase in the compound that is observed to be superconducting, suggesting the important role played by the lattice strain towards superconductivity. Despite the orthorhombic phase not exhibiting superconductivity, we observe a significant increase in the lattice strain as the temperature approaches the TC . In addition, the particle size appears to play an important role in the stabilisation of structural phase separation in the case of BPBO75. This underscores the importance of sample preparation techniques as a tool to tune superconductivity in this compound.
Figure 4. Variation of orthorhombic strain for BPBO70 (open squares), BPBO75 (open circles), and BPO (open rhombus) compounds. Panels (a)–(c) show the orthorhombic strain in the  ,
,  and bc-planes of the unit cell. The illustration on the right side of the panels highlights the apical and the basal bonds present in the compound. Dashed lines are a visual guide indicating the overall behaviour of the various parameters.
and bc-planes of the unit cell. The illustration on the right side of the panels highlights the apical and the basal bonds present in the compound. Dashed lines are a visual guide indicating the overall behaviour of the various parameters.
Download figure:
Standard image High-resolution image4.3. Electronic structure of BPBO
4.3.1. XPS and band structure calculations.
To understand the evolution of the electronic structure of the BPBO with increasing Pb content, we have performed electronic structure calculations. Figure 5 shows the total DOS and partial DOS for the three compositions. Both BPBO70, and BPBO75 lie in the region of metal-semiconductor transition. As a result of this, it is reasonable to expect both metallic and semiconducting regions in the sample. Thus, to account for this, we have performed TB mBJ calculations for these two compounds. Further more, the BPBO75 compound is diphasic, and to account for it, the calculations were performed on both the phases, and have been added in 70:30 ratio (tetragonal:orthorhombic), as was observed experimentally [22]. When the TB-mBJ calculations for BPBO70 and BPBO75 are compared, we observe an additional feature B0 in the latter. This feature arises because of the increased orthorhombicity. The structural parameters of the orthorhombic phase of BPBO70 are closer to tetragonal crystal structure—the difference between a and b lattice parameters is very small, ( Å). The structural parameters of the orthorhombic phase of BPBO75, on the other hand, have a distinct difference between the a and b lattice parameters, i.e.
Å). The structural parameters of the orthorhombic phase of BPBO75, on the other hand, have a distinct difference between the a and b lattice parameters, i.e.  Å. This structural distinction between the orthorhombic phases of the two compounds, namely BPBO70 and BPBO75, are reflected in their electronic structure as well.
Å. This structural distinction between the orthorhombic phases of the two compounds, namely BPBO70 and BPBO75, are reflected in their electronic structure as well.
Figure 5. Calculated total and partial DOS for BPBO (BPBO70 (A1-A6), BPBO75 (B1-B6) and BPO (C1-C6)) obtained from TB mBJ (for BPBO70, BPBO75), and LDA (for BPO). In panel C1, LDA represents the total DOS obtained from the calculation where an oxygen vacancy was introduced.
represents the total DOS obtained from the calculation where an oxygen vacancy was introduced.
Download figure:
Standard image High-resolution imageThe end compound, on the other hand, has been reported [6, 9, 10, 18, 21] to be metallic. However, our LDA calculations show a finite gap at the Fermi level. The origin of the metallicity in this compound is an open question. One of the mechanisms suggested to be at work by Ikushima and Hayakawa [9] and Shannon and Bierstedt [10] is by means of oxygen vacancies. To ascertain whether oxygen deficiency plays any role in this compound, we performed electronic structure calculations using LDA, and an oxygen vacancy of 8.3% was introduced. We observe that introducing oxygen vacancy results in a finite DOS at Fermi level, as seen in panel C1 of figure 5, thus indicating to some extent that oxygen vacancies play a role in the metallic behaviour of this compound. The presence of oxygen vacancies can be confirmed using core level studies.
To understand the behaviour of the valence band spectra, XPS measurements were carried out on the compounds under study at 300 K; figure 6(a) shows the XPS valence band spectra of these compounds. The comparison of XPS and He valence band spectra collected at 300 K is shown in figures 6(b)–(d). The inset in panel (a) of the figure shows finite DOS at EF
for all three compounds. In all the compounds, we observe six features in the spectra labelled as A, B1, B2, B3, C and D (see figures 6(b)–(d)). To ascertain the presence of feature B0 in BPBO75, the intensity ratio corresponding to the features around −2 eV to −1.35 eV in BPBO70 and BPBO75 was calculated. Our results show that in the case of BPBO70, it is around 1.9 and BPBO75, it is around 4.3. This confirms the presence of the feature B0 in BPBO75. In table 2, various contributions that make up the features are listed.
valence band spectra collected at 300 K is shown in figures 6(b)–(d). The inset in panel (a) of the figure shows finite DOS at EF
for all three compounds. In all the compounds, we observe six features in the spectra labelled as A, B1, B2, B3, C and D (see figures 6(b)–(d)). To ascertain the presence of feature B0 in BPBO75, the intensity ratio corresponding to the features around −2 eV to −1.35 eV in BPBO70 and BPBO75 was calculated. Our results show that in the case of BPBO70, it is around 1.9 and BPBO75, it is around 4.3. This confirms the presence of the feature B0 in BPBO75. In table 2, various contributions that make up the features are listed.
Figure 6. (a) Comparison of Al K spectrum with composition, BPBO70 (black), BPBO75 (red), BPO (blue). (b)–(d) Comparison of Al K
spectrum with composition, BPBO70 (black), BPBO75 (red), BPO (blue). (b)–(d) Comparison of Al K spectrum (open circles, yellow), He
spectrum (open circles, yellow), He spectrum (solid line, purple) and total DOS (solid line, orange) obtained from TB mBJ (for BPBO70, BPBO75) and LDA (for BPO). The total DOS (TDOS) has been shifted by −1.339 eV for BPBO70 compound, −1.0 eV for BPBO75 compound, and −0.49 eV for BPO compound to match the experimental spectra. The calculations have been carried out in the orthorhombic phase for BPBO70, and BPO, and in tetragonal and orthorhombic phases for BPBO75 which were then added in 70:30 ratio (tetragonal:orthorhombic) to obtain the resultant data.
spectrum (solid line, purple) and total DOS (solid line, orange) obtained from TB mBJ (for BPBO70, BPBO75) and LDA (for BPO). The total DOS (TDOS) has been shifted by −1.339 eV for BPBO70 compound, −1.0 eV for BPBO75 compound, and −0.49 eV for BPO compound to match the experimental spectra. The calculations have been carried out in the orthorhombic phase for BPBO70, and BPO, and in tetragonal and orthorhombic phases for BPBO75 which were then added in 70:30 ratio (tetragonal:orthorhombic) to obtain the resultant data.
Download figure:
Standard image High-resolution imageTable 2. Contributions to various features A, B0, B1, B2, B3 and C. The intensity of feature D is mainly due to surface oxygen states.
| Contribution | |||
|---|---|---|---|
| Feature | BPBO70 | BPBO75 | BPO |
| A | Bi 6s, Pb 6s | Bi 6s, Pb 6s | Pb 6s, O 2p; |
| O 2p | O 2p | Pb 6p, 6d (weak) | |
| B0 | — | Bi/Pb 6d, 5f, O 2p; | — |
| Bi/Pb 6p (weak) | |||
| B1 | Bi/Pb 6p, 6d | Bi/Pb 6p, 6d, 5f | Pb 6d, 5f, O 2p; |
| 5f, O 2p | O 2p | Pb 6s, 6p (weak) | |
| B2 | Bi/Pb 6p, 6d, 5f | Bi/Pb 6p, 6d, 5f | Pb 6p, 6d, 5f |
| O 2p; Bi 6s (weak) | O 2p; Bi 6s (weak) | O 2p; Pb 6s (weak) | |
| B3 | Bi/Pb 6p, 6d, O 2p; | Bi/Pb 6s, 6p, 6d | Pb 6s, 6p, 6d |
| Bi/Pb 6s, 5f (weak) | 5f (weak), O 2p | 5f, O 2p | |
| C | Bi/Pb 6p, O 2p; | Bi/Pb 6p, O 2p; | Pb 6p, O 2p; |
| Bi/Pb 6d, 5f (weak) | Bi/Pb 6d, 5f (weak) | Pb 6d, 5f (weak) | |
Feature D represents the surface cleanliness; its intensity in XPS spectrum is lower compared to the He spectrum due to the higher sensitivity of the He
spectrum due to the higher sensitivity of the He radiation to the surface oxygen. We can clearly observe from the table, that despite the similarity in the electronic structure, the origin of the intensities in different regions is different, thus indicating the dependence on the crystal structure of the compound.
radiation to the surface oxygen. We can clearly observe from the table, that despite the similarity in the electronic structure, the origin of the intensities in different regions is different, thus indicating the dependence on the crystal structure of the compound.
Figures 7(a)–(e) shows the XPS spectrum of various core levels of BPBO (BPBO70 (black), BPBO75 (red) and BPO (blue)) at RT.
Figure 7. (a)–(e) Various core level XPS spectra of BPBO70 (black), BPBO75 (red) and BPO (blue) collected at 300 K: (a) O 1s (b) Bi  and (c) Pb
and (c) Pb  (d) Ba
(d) Ba  (e) Ba
(e) Ba  . The labels A,
. The labels A,  and
and  in panel (a) indicate the lattice oxygen, oxygen vacancy, and surface oxygen contribution to the oxygen 1s spectrum, respectively; the labels B and
in panel (a) indicate the lattice oxygen, oxygen vacancy, and surface oxygen contribution to the oxygen 1s spectrum, respectively; the labels B and  in panels (d) and (e) indicate the positions of poorly-screened and well-screened peaks, respectively. Panel (f) shows the comparison of normalised valence band spectra around EF
at 30 K and panel (g) the SDOS of
in panels (d) and (e) indicate the positions of poorly-screened and well-screened peaks, respectively. Panel (f) shows the comparison of normalised valence band spectra around EF
at 30 K and panel (g) the SDOS of  and 1.0 collected at 30 K. Panels (h) and (i) show the evolution of SDOS of BPBO70 and BPO compounds with temperature. The inset to panel (f) shows the intensity close to the Fermi level at 300 K.
and 1.0 collected at 30 K. Panels (h) and (i) show the evolution of SDOS of BPBO70 and BPO compounds with temperature. The inset to panel (f) shows the intensity close to the Fermi level at 300 K.
Download figure:
Standard image High-resolution imageIn the case of O 1s spectra, the most intense feature A corresponds to the contribution arising from the oxygen ions in the bulk. The feature  around 529.5 eV is attributed to oxygen vacancies [35] and the feature
around 529.5 eV is attributed to oxygen vacancies [35] and the feature  is attributed to contribution of surface oxygen and is observed around 531 eV. The contribution of feature
is attributed to contribution of surface oxygen and is observed around 531 eV. The contribution of feature  is significant in the case of the end compound as compared to the rest of the compounds suggesting a significant role played by oxygen vacancies. This behaviour is in line with our electronic structure calculations where introduction of oxygen vacancies gives rise to metallic behaviour in the end compound.
is significant in the case of the end compound as compared to the rest of the compounds suggesting a significant role played by oxygen vacancies. This behaviour is in line with our electronic structure calculations where introduction of oxygen vacancies gives rise to metallic behaviour in the end compound.
The Bi and Pb 4 peaks exhibit twin features labelled as B and
peaks exhibit twin features labelled as B and  . These features are attributed to poorly screened and well screened final states, respectively. The well screened feature arises when the Bi/Pb 4
. These features are attributed to poorly screened and well screened final states, respectively. The well screened feature arises when the Bi/Pb 4 hole is screened by the transfer of electrons from the ligand and the poorly screened feature arises when no such transfer occurs. The intensity ratio of feature
hole is screened by the transfer of electrons from the ligand and the poorly screened feature arises when no such transfer occurs. The intensity ratio of feature  to B is the least in BPBO70, and is nearly thrice as large in case of the other two compounds. It is important to note that the separation between the features B to
to B is the least in BPBO70, and is nearly thrice as large in case of the other two compounds. It is important to note that the separation between the features B to  in binding energy and its relative intensity depends on the charge transfer energy and the hybridisation between the O 2p and Bi/Pb site 4
in binding energy and its relative intensity depends on the charge transfer energy and the hybridisation between the O 2p and Bi/Pb site 4 hybridisation. The high intensity of the feature B as compared to
hybridisation. The high intensity of the feature B as compared to  in BPBO70 compound suggests weak hybridisation between the above two sites as compared to the rest of the compounds.
in BPBO70 compound suggests weak hybridisation between the above two sites as compared to the rest of the compounds.
The width and the asymmetry of the Ba core levels of the end compound is maximum as compared to the rest of the compounds. The behaviour of the asymmetry suggests that the density of states at the Fermi level in the case of the end compound is expected to be more as compared to the rest of the compounds, as observed in the XPS valence band spectra, inset of figure 6(a). This aspect is also observed in behaviour of room temperature valence band spectra close to the Fermi level using high resolution UPS.
Apart from the behaviours of the relative intensities of the features, the shift in the binding energy of the core levels as a function of composition carries vital information about the properties of the systems. The shift in the binding energy can be written as  ; where µ, VM
,
; where µ, VM
,  and ER
correspond to the chemical potential shift, Madelung potential, chemical shift and extra atomic relaxation energy of the core hole state, respectively.
and ER
correspond to the chemical potential shift, Madelung potential, chemical shift and extra atomic relaxation energy of the core hole state, respectively.
For the case of BPBO70, BPBO75 and the end compounds, the average Ba–O bond distance obtained from the Rietveld analysis is around 3.07 Å, 3.04 Å and 3.03 Å, respectively. From this behaviour, it is expected that the Ba core levels of the end compound to be shifted towards higher binding energy as compared to the rest of the compounds. But we observe opposite behaviour. Hence, the role of the terms other than  in the above equation are expected to be at play. However, it is important to note that Ba–O bonds being ionic in nature, the contributions due to
in the above equation are expected to be at play. However, it is important to note that Ba–O bonds being ionic in nature, the contributions due to  and
and  are insignificant. Hence, the shift of the Ba core levels towards lower binding energy suggests the role played by the shift in the chemical potential towards the valence band as the concentration of Pb increases from 75% in BPBO75 to 100% in the end compound.
are insignificant. Hence, the shift of the Ba core levels towards lower binding energy suggests the role played by the shift in the chemical potential towards the valence band as the concentration of Pb increases from 75% in BPBO75 to 100% in the end compound.
4.3.2. UPS valence band studies near EF .
Let us now look into the evolution of the states close to EF
with increase in Pb doping. In panel (f) of figure 7, we show the near-EF
valence band spectra of the three compounds under study collected using He spectra at 30 K; the inset shows the region very close to fermi edge at 300 K. We observe a finite DOS at EF
for all three compounds. At lowest collected temperature (30 K), at the EF
, the intensity is the highest in the case of end compound followed by BPBO70 and then BPBO75, figure 7(f). To obtain the evolution of the states around EF
with temperature, spectral density of states (SDOS) was plotted. The SDOS was obtained by dividing the experimental spectra by the Fermi Dirac distribution and symmetrising it. Panel (g) shows the SDOS for the three compounds; the dip of the SDOS deepens with reducing temperature. This is typically an indication of pseudogap existing in the compound, as was in the case of BPBO75 compound [22]. Whereas in BPBO70 and BPBO75 [22] the behaviour is very similar, i.e. a large change in the intensity of the SDOS around EF
with reducing temperature, in case of the end compound, we observe a small reduction in the intensity of SDOS around EF
as temperature decreases until 50 K, below which a drastic drop can be seen. To ascertain the origin of this pseudogap, we have fit the the SDOS at various temperatures with the function
spectra at 30 K; the inset shows the region very close to fermi edge at 300 K. We observe a finite DOS at EF
for all three compounds. At lowest collected temperature (30 K), at the EF
, the intensity is the highest in the case of end compound followed by BPBO70 and then BPBO75, figure 7(f). To obtain the evolution of the states around EF
with temperature, spectral density of states (SDOS) was plotted. The SDOS was obtained by dividing the experimental spectra by the Fermi Dirac distribution and symmetrising it. Panel (g) shows the SDOS for the three compounds; the dip of the SDOS deepens with reducing temperature. This is typically an indication of pseudogap existing in the compound, as was in the case of BPBO75 compound [22]. Whereas in BPBO70 and BPBO75 [22] the behaviour is very similar, i.e. a large change in the intensity of the SDOS around EF
with reducing temperature, in case of the end compound, we observe a small reduction in the intensity of SDOS around EF
as temperature decreases until 50 K, below which a drastic drop can be seen. To ascertain the origin of this pseudogap, we have fit the the SDOS at various temperatures with the function  . At 30 K, we obtain α = 0.77 for BPBO70, α = 0.5 for BPBO75 [22] and α = 0.65 for BPO. Typically, when disorder plays a dominant role, the value of α is observed to be 0.5; the deviation from the ideal value of 0.5 indicates that there are other effects that contribute to the pseudogap, in addition to disorder and warrants further study for better understanding of the origin of the pseudogap. However, the origin of disorder in the three compounds appear to be distinct from each other. For the BPBO70 compound, the origin of disorder is due the presence of the metallic and semiconducting regions in the sample. In the case of BPBO75 compound, both structural dimorphism and coexistence of metallic and semiconducting regions in the sample contribute to the disorder in the compound [22]. Finally, in case of the end compound, the oxygen vacancies would play a major role in the origin of disorder.
. At 30 K, we obtain α = 0.77 for BPBO70, α = 0.5 for BPBO75 [22] and α = 0.65 for BPO. Typically, when disorder plays a dominant role, the value of α is observed to be 0.5; the deviation from the ideal value of 0.5 indicates that there are other effects that contribute to the pseudogap, in addition to disorder and warrants further study for better understanding of the origin of the pseudogap. However, the origin of disorder in the three compounds appear to be distinct from each other. For the BPBO70 compound, the origin of disorder is due the presence of the metallic and semiconducting regions in the sample. In the case of BPBO75 compound, both structural dimorphism and coexistence of metallic and semiconducting regions in the sample contribute to the disorder in the compound [22]. Finally, in case of the end compound, the oxygen vacancies would play a major role in the origin of disorder.
It is very important to note that despite the absence of superconductivity in BPBO70, we observe a pseudogap at low temperatures. Furthermore, a pseudogap has been observed in the case of the non-superconducting BPO as well. This suggests that this pseudogap is not necessarily linked to superconductivity, although it is expected to play an important role in the onset of superconductivity. Our results show that it is very important to study the microscopic origins of the pseudogap and disorder as they are likely to play different roles on different compositions. In general, in cuprate superconductors, the pseudogap acts as a precursor to superconductivity [16]. However, in the temperature doping phase diagram of electronic properties of BPBO [8, 36], transition from metallic to superconductivity has been reported. Our results call for a revision in the phase diagram of BPBO to include the pseudogap as an important feature that exists before the onset of superconductivity, both as a function of temperature and composition.
5. Summary
In conclusion, we have studied the crystal and electronic structure of the orthorhombic phases of the polycrystalline BPBO for BPBO70, BPBO75 and BPO. The difference in sample preparation techniques adopted for BPBO70 and BPBO75 compounds suggests the crucial role played by the particle size in stabilising superconductivity. The temperature-dependent XRD measurements indicate that the BPBO70 is monophasic, despite lying in the superconducting compositional region, whereas the BPBO75 exhibits the expected diphasic behaviour. The end compound remains in the orthorhombic Ibmm crystal structure down to 10 K. The BPBO70 compound exhibits semiconductor-like behaviour in the resistivity measurements despite showing a superconducting transition in the magnetic measurements. The behaviour of the orthorhombic strain of BPBO70 and BPBO75 compounds in the ab-plane indicates that the strain acts as a precursor to superconductivity observed in the BPBO. Our core level studies reveal the signature of metallicity with increase in x. The near-EF UPS valence band studies indicate the presence of a pseudogap. Our results suggest that effects other than disorder contribute to the pseudogap in BPBO70 and BPO. The DFT calculations along with photoemission spectroscopy measurements reveal that oxygen vacancies present in the end compound play a major role in the origin of metallicity. Our results demand further investigations to understand the effects of particle size, origin of pseudogap, nature of charge carriers, compositions at the grain boundaries and the electronic structure in the superconducting phase. Furthermore, our studies will help in refining the temperature-doping phase diagram of BPBO.
Acknowledgments
The authors, Bharath M and R Bindu thank Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India for funding this work. This work is funded under the SERB project sanction order No. EMR-2016-001144.
Data availability statement
The data cannot be made publicly available upon publication because they are not available in a format that is sufficiently accessible or reusable by other researchers. The data that support the findings of this study are available upon reasonable request from the authors.








