Abstract
Ar or O2 fine bubbles of diameter <80 μm were introduced in water and a pulsed discharge plasma was generated between cylinder electrodes in water. Fine bubbles in water affected discharge ignition and caused low inception voltage and suppression of rising temperature. The contamination from electrodes was suppressed in the case of fine bubbles addition because fine bubbles assisted plasma generation. In addition, discharge with fine bubbles enhanced plasma emission with high electron density compared to the no-bubbling case. Discharge with fine bubbles at low-pH conditions generated intense plasma emission compared to neutral and high-pH conditions owing to the electric charge of the fine bubbles.
Export citation and abstract BibTeX RIS
1. Introduction
Electric discharge in the presence of water has potential use in chemical processing because it can induce chemical reactions rapidly without the need to use catalysts or chemical agents. In general, the main reactions induced by electric discharge with water are oxidizing because the discharge plasma generates oxidation species such as the OH radical, oxygen atom, ozone, and hydrogen peroxide [1–3]. Therefore, a variety of applications using discharge plasma in water have been examined, including water purification [4, 5], sterilization [6], and nanomaterial synthesis [7]. However, high voltage is required to initiate breakdown and sustain discharge plasma in the liquid phase compared with the gas phase [8, 9]. There are several research reports on discharge plasma in the gas phase in contact with water [10, 11]. Although it is much easier to generate plasma using an electric discharge in the gas phase over a liquid surface than within the liquid phase, the contact area between the discharge plasma and the liquid is small.
Recently, several discharge methods using bubbles in water have been proposed to form discharge plasmas at low voltage with a large contact area [8, 12, 13]. Moreover, discharge in bubbles could produce reactive species derived from the introduction of gas molecules [14, 15]. Some researchers have reported that discharge in bubbles reduced energy consumption [16, 17], promoted the generation of active species [8], and increased the reaction rate [14].
In this work we utilized fine bubbles for electric discharge in water. A fine bubble is a bubble whose diameter is <100 μm, namely a microbubble or nanobubble. There are differences in behavior in water between millibubbles and fine bubbles. Millibubbles rise rapidly in water and finally burst at the surface of the water. In contrast, fine bubbles of 50 μm in diameter exhibit extremely slow rising speeds of 1 mm s−1. During bubble rising, fine bubbles decrease in size and finally collapse in water [19, 20].
Almost all of previous discharge methods with bubbles introduced bubbles on the order of a millimeter from a nozzle set in water [15, 16, 18]. In these methods, bubbles rose one after another owing to the high rising speed of the millibubbles, and the methods consumed a lot of gas. Using fine bubbles for introducing gas reduces gas consumption compared to experiments with millibubbles. Additionally, some reactive species produced by the discharge plasma were supposed to exist inside the bubbles. Although millibubbles including reactive species escape from the water, fine bubbles are expected to retain chemical active species for a long time in water owing to their slow rising speed, leading to enhanced reactivity.
In this paper, a pulsed discharge plasma was generated in water with argon (Ar) or oxygen (O2) fine bubbles. To examine the effects of fine bubbles on the characteristics of the electric discharge in water, we investigated the electrical properties and optical emission from the discharge plasma in water with fine bubbles.
2. Experimental apparatus
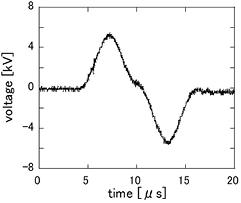
The experimental setup is schematically shown in figure 1. Two cylindrical 1.0 mm-diameter copper electrodes were set at a distance of 0.7 mm apart in a reactor vessel. The 67 mm-diameter vessel was filled with 250 mL of distilled water. Fine bubbles were generated by a micro–nano bubble generator (MA3, Asupu Co., Ltd.) with water circulated at a flow rate of 150 mL min−1. In the case of the discharge in water without bubbles, water was circulated at the same rate. Feed gas (Ar or O2) was introduced into micro–nano bubble generator at a flow rate of 30 mL min−1 and mixed with the water. The injection nozzle for water with fine bubbles was located 5 cm above the discharge region to prevent millibubbles from existing between electrodes. The picture shown at the upper right of figure 1 presents the situation of discharge area at the beginning of fine bubble addition. A bipolar pulsed voltage of ±5–12 kV shown in figure 2 was applied to one electrode by a pulse power supply (TE-HVP1510K300-NP, Tamaoki Electronics Co., Ltd.); the other was grounded. The frequency of the wave pulse was 10 kHz. Pulse power was operated by a trigger control (AFG-2005, Good Will Instrument Co., Ltd.), whose frequency and duty ratio were 20 Hz and 10%, respectively, to avoid erosion of the electrodes. The power was applied after filling fine bubbles fully in the reactor.
Figure 1. Schematic of the experimental setup.
Download figure:
Standard image High-resolution imageFigure 2. Waveform of bipolar pulsed voltage.
Download figure:
Standard image High-resolution imageThe diameter and density of fine bubbles were measured using shadowgraph imaging by an intensified charge-coupled device camera (Roper Scientific Inc.) [21]. Discharge voltage and current were monitored using an oscilloscope (TDS2024C, Tektronix Inc.) with a high-voltage probe (EP-50K, Nissin Pulse Electronics Co. Ltd.). Water temperatures were monitored at a distance of 20 mm from the discharge area during discharge by a digital temperature indicator. Water qualities before and after discharge were evaluated by a conductivity meter (AS710, Asone Co.), an inductively coupled plasma optical emission spectrometry (ICP-OES; IRIS-AP Thermo Fisher Scientific), and a dissolved oxygen meter (PDO-520, Fuso Co., Ltd.). To observe plasma emission, optical emission spectra were obtained by using a high-resolution spectrometer (HR4000, Ocean Optics Inc.).
3. Results and discussion
The diameter of the fine bubbles was found to be less than 80 μm from the shadowgraph image. Additionally, the number density of fine bubbles was  m−3. In other words, about 50 fine bubbles existed between the two electrodes in ϕ
m−3. In other words, about 50 fine bubbles existed between the two electrodes in ϕ  mm.
mm.
The pulsed discharge plasma was generated in distilled water with and without fine bubbles. Figure 3 shows the discharge inception voltage obtained by using bipolar pulsed power with and without Ar or O2 fine bubbles. Discharge inception voltages decreased by 50% with argon bubbling and by 25% with oxygen bubbling compared with the no-bubbling case. Thus, the addition of fine bubbles made it easier to generate an electric discharge.
Figure 3. Discharge inception voltage with and without fine bubbles.
Download figure:
Standard image High-resolution imageThe water temperature after the discharge at 9.5 kV is shown in figure 4. The water temperature before the discharge was controlled to be at 20 °C ± 2 °C. After treatment of the discharge without bubbles for 30 min, the water temperature increased steadily. In contrast, the discharge in water with fine bubbles restrained the rise in temperature.
Figure 4. Temperature changes of water after treatment of pulsed discharge at 9.5 kV.
Download figure:
Standard image High-resolution imageDifferences between no bubbling and fine bubbling were probably caused by the discharge ignition. Given the breakdown initiation in water without bubbles, thermal bubbles were created on the tips of the electrodes by Joule heating when the voltage was applied to the electrodes in water. The electric field was enhanced inside the bubbles created by Joule heating and this initiates breakdown because the gas phase requires smaller electric fields than the liquid phase when discharge plasma generated in water without bubbles [22, 23]. In the case of discharge in water with fine bubbles, the fine bubbles that attached to the electrodes were assumed to lead the breakdown. As other possibility, it will be considered that the electric field is increased by creating cusps at the contact point between two bubbles when a lot of bubbles exist in the gap. However, it was unlikely to induce breakdown in our case because fine bubbles repulsed each other in water due to surface charges [24, 25]. Because thermal bubbles created by Joule heating are thus not needed when the discharge plasma was generated with fine bubbles, this led to the low discharge inception voltage and suppression of water temperature increase. In addition, the discharge inception voltage depended on the introduced gas because the discharge ignition probably occurred inside the bubbles and the degree of ionization was different.
To evaluate water properties after discharge, the copper concentration of water after discharge at ±9.5 kV were analyzed by ICP-OES. The copper concentration in water before the discharge was <0.05 ppm. After discharge with no bubbling for 30 min, the copper concentration rose to 2.5 ppm. In contrast, water properties after the discharge with Ar bubbling in 30 min did not change, remaining at <0.05 ppm. These results considerably affect the electric field on the electrode surfaces, and strong electric fields contribute to electrodes erosion [26, 27]. The electric field of the discharge in water with no bubbling is of the order of 109 V m−1, compared to 106 V m−1 in the gas phase [8]. When fine bubbles assisted discharge ignition, electric field was supposed to be lower than no bubbling and Joule heating of electrodes were suppressed. Thus, water after the discharge with fine bubbles was contaminated slightly with copper.
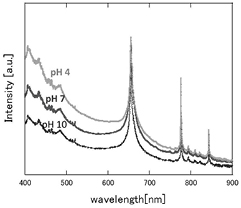
Optical emission spectra were observed to investigate the characteristics of plasma emission. Figure 5 shows the optical emission spectra for the discharge plasma generated in distilled water with and without fine bubbles when the discharge was applied at 9.5 kV. Hydrogen and oxygen atomic lines from dissociation of H2O molecules were dominant in the spectra. Fine bubbles significantly enhanced plasma emission, and Hβ (486 nm), Hγ (434 nm), and Hδ (410 nm) lines, which are high energy levels of hydrogen atoms, were also observed. These observations could likely be attributed to the lower breakdown voltage by the fine bubbles [13]. Intensive broad emissions were mainly attributed to the dynamic Stark effect of Hβ, Hγ and Hδ [28]. Recombination of water molecules [29], dissociation of hydrogen [30], and bremsstrahlung [31, 32] also will be considered as the cause of broad emissions in the shorter wavelength region. However, emission of argon was not observed even in the discharge with Ar fine bubbling, indicating that the emission of the discharge plasma with fine bubbles derived from water molecules and not from Ar gas.
Figure 5. Optical emission spectra with and without fine bubbles when the discharge was applied at 9.5 kV.
Download figure:
Standard image High-resolution imageThe electron density was estimated from the Stark effect of the Hα (656 nm) line from optical emission spectra [33, 34]. Table 1 lists energy consumption calculated by voltage and current wavelengths, electron density and the intensity ratio of O (777 nm) and Hα (656 nm) lines from the optical emission spectra in figure 5. The energy consumption per pulse of the discharge without bubbles was lower than that with fine bubbles because the energy was consumed by generation of Joule heating. The electron density of the discharge plasma with fine bubbling is about an order of magnitude higher than in the case with no bubbling. Meanwhile, the intensity ratio of O/Hα of Ar bubbling was almost the same as in the no bubbling case.
Table 1. Energy consumption calculated by voltage and current waveforms, electron density and intensity ratio of O (777 nm) and Hα (656 nm) estimated from optical emission in figure 5.
| Energy consumption [mJ/pulse] | Electron density [cm−3] | Intensity ratio of O (777 nm)/Hα (656 nm) | |
|---|---|---|---|
| No bubbling | 21.9 | 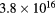 |
0.47 |
| Ar bubbling | 105.2 | 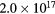 |
0.52 |
| O2 bubbling | 250.4 | 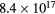 |
0.90 |
The electron density in the case of O2 bubbling was a factor of 4 higher than that with Ar bubbling. Additionally, the intensity ratio of O/Hα with O2 bubbling was higher than that with Ar bubbling. This difference could be attributed to the amount of dissolved oxygen in water; when O2 gas was bubbling in the water, there was a high concentration of oxygen dissolved in the water [35]. Our experiment also indicated that dissolved oxygen in water increased to 23 mg L−1 with O2 fine bubbling from 4 mg L−1 with no bubbling. At this time, the conductivity of water after O2 fine bubbling rose to 1.7 μS cm−1 from 1.5 μS cm−1 of no-bubbling case in spite of no change of Ar fine bubbling. These values were measured after removing fine bubbles in water. Therefore, the rising of the conductivity was supposed to be caused by dissolved oxygen in water. Although the conductivity cannot measure in a state of including bubbles in water, the conductivity of the water during O2 fine bubbling is expected to be higher than Ar fine bubbling. It leaded to high electron density in the case of O2 fine bubbling because high conductivity induced broad optical emission [28, 36]. Moreover, high concentration of dissolved oxygen in water presumably created more oxygen species which promoted dissociation of H2O molecules [3, 37], and the discharge with O2 fine bubbling indicated intense plasma emission compared to Ar fine bubbling.
The electric charge on the fine bubble surface varied widely depending on the pH value of the aqueous solution [19]. To examine the effects on electric charge on the fine bubbles, discharge plasmas were generated in aqueous solutions at several pH values. The pH of the aqueous solution was adjusted by the addition of HCl, NaCl, and NaOH to distilled water. The conductivity of the aqueous solution was controlled at 90 μS cm−1. The pH dependence of plasma emission with and without fine bubbles is shown in figures 6 and 7. Emission peaks of sodium and chlorine from the solute are not found in either figure. The optical emission spectra of the electric discharge without bubbles were barely influenced by the pH value of the aqueous solution, as shown in figure 6. Table 2 lists the intensity ratio of the O (777 nm) and Hα (656 nm) lines on figures 6 and 7. The electron density at pH 10 was slightly higher than at pH 7 and pH 4. In contrast, the intensity ratio of O/Hα was approximately the same value at any pH condition for discharge without bubbles.
Figure 6. pH dependence of optical emission spectra of discharge with no bubbling.
Download figure:
Standard image High-resolution imageFigure 7. pH dependence of optical emission spectra of discharge with Ar bubbling.
Download figure:
Standard image High-resolution imageTable 2. Intensity ratio of O (777 nm) and Hα (656 nm) from optical emission in figures 6 and 7.
| pH value | Electron density [cm−3] | Intensity ratio of O (777 nm)/Hα (656 nm) | |
|---|---|---|---|
| no bubbling | pH 10 | 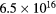 |
0.54 |
| pH 7 | 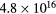 |
0.53 | |
| pH 4 | 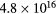 |
0.49 | |
| Ar bubbling | pH 10 | 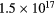 |
0.64 |
| pH 7 | 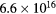 |
0.54 | |
| pH 4 | 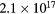 |
0.70 |
In contrast, pH values did influence the emission spectra when the discharge plasmas were generated with Ar fine bubbles. The aqueous solution with fine bubbles at low pH induced strong emission, especially in the shorter wavelength region. Inspection of the intensity ratio of O/Hα in table 2 indicates that the intensity ratio for the discharge with Ar fine bubbling at pH 7 was the same as that with no bubbling, but intensities of oxygen in Ar bubbling were stronger relatively at pH 4 and pH 10. In strong acid and basic conditions, high electron density was achieved by discharge in water. In our experiments discharge plasmas were more easily generated under high-pH conditions. This caused high electron density by electric discharge regardless of the presence of fine bubbles at pH 10, as indicated in table 2. When the discharge plasma was generated at pH 4 with Ar fine bubbles, the electric charge on fine bubble surface probably affected plasma generation because the electric charge of the solute generally influenced plasma emission [38]. Fine bubbles also have an electric charge on the interface between the bubble surfaces and the aqueous solution. ζ potential, which is electric potential in the interfacial double layer, of a fine bubble in aqueous solution at neutral and basic conditions is negative. However, ζ potential of fine bubbles is positive in acidic condition below pH 4.5 [24, 25]. In other words, there are many negative ions around the bubble surface at pH 4. Considering an early stage of plasma generation inside the bubble, which attached the electrode, an initial discharge was generated from the electrode toward bubble surface. At this time, the surface water layer inside the bubble was evaporated, and the plasma was propagated toward the other electrode. When the bubble with surface charge became a trigger of plasma generation, vapor including a lot of H+ or OH− ions was filled inside the bubble after the initial discharge. Since H+ and OH− ions give extremely high conductivity [39], it is supposed to be easy to form plasma inside the bubble when bubbles have surface charge. We speculate that it caused the difference of electron densities in the case of Ar fine bubbling shown in table 2.
Moreover, the higher electron density resulted in the larger intensity ratio of O/Hα obtained. Although we have no results on the energy distribution of electrons, the O atom with its lower excitation energy compared with the H atom should be more easily emitted. Namely, the increase of the O/Hα ratio with increasing electron density would be caused by the increase in the intensity of the O line.
4. Conclusion
Pulsed discharge plasmas in water with fine bubbles were generated by bipolar pulsed power. We examined the effects of fine bubbles on the discharge plasmas. Fine bubbles played a role in discharge initiation as an aid to breakdown, lowering the discharge inception voltage and avoiding Joule heating. Discharge plasmas with fine bubbles indicated low contamination from electrodes owing to the low electric field on electrode surfaces. Moreover, fine bubbles enhanced plasma emission of hydrogen and oxygen atoms, which derived from dissociation of water molecules, but optical emission from feed gas was not observed. Discharge plasmas with fine bubbles especially with high electron density emitted hydrogen atoms with high energy levels. Dissolved oxygen in water strengthened the emission of oxygen atoms and produced discharge plasma with high electron density. The electric charge of fine bubbles also affected plasma emission. Plasma emission became strong and high electron density was observed for discharge in low-pH solutions with fine bubbles because initial electrons could easily be supplied from the fine bubble surfaces, which were negatively charged.
Acknowledgment
This work was supported by the Grants-in-Aid for Scientific Research on 'Frontier Science of Interactions between Plasma and Nano Interfaces' (No. 21110009) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.