Abstract
In the last 40 years a wide range of molecules, including neutrals, cations and anions, containing up to 13 atoms—in addition to detections of  and
and  —have been found in the harsh environment of the interstellar medium. The exquisite sensitivity and very high spectral and, more recently, spatial resolution, of modern telescopes has enabled the physics of star formation to be probed through rotational line emission. In this article, I review the basic properties of interstellar clouds and the processes that initiate the chemistry and generate chemical complexity, particularly in regions of star and planet formation. Our understanding of astrochemistry has evolved over the years. Before 1990, the general consensus was that molecules were formed in binary, gas-phase, or volume, reactions, most importantly ion-neutral reactions despite the very low ionization in clouds. Since then, observations have indicated unambiguously that there is also a contribution from surface processes, particularly on the icy mantles that form around refractory grain cores in cold, dense gas. The balance between these two processes depends on particular physical conditions and can vary during the life cycle of a particular volume of interstellar cloud.
—have been found in the harsh environment of the interstellar medium. The exquisite sensitivity and very high spectral and, more recently, spatial resolution, of modern telescopes has enabled the physics of star formation to be probed through rotational line emission. In this article, I review the basic properties of interstellar clouds and the processes that initiate the chemistry and generate chemical complexity, particularly in regions of star and planet formation. Our understanding of astrochemistry has evolved over the years. Before 1990, the general consensus was that molecules were formed in binary, gas-phase, or volume, reactions, most importantly ion-neutral reactions despite the very low ionization in clouds. Since then, observations have indicated unambiguously that there is also a contribution from surface processes, particularly on the icy mantles that form around refractory grain cores in cold, dense gas. The balance between these two processes depends on particular physical conditions and can vary during the life cycle of a particular volume of interstellar cloud.
The complex chemistry that occurs in space is driven mostly through interaction of the gas with cosmic ray protons, a source of ionization that enables a rich ion-neutral chemistry. In addition, I show that the interaction between the gas and the dust in cold, dense regions also leads to additional chemical complexity through reactions that take place in ices at only a few tens of degrees above absolute zero. Although densities are low compared to those in terrestrial environments, the extremely long life times of interstellar clouds and their enormous sizes, enable complex molecules to be synthesised and detected. I show that in some instances, particularly in reactions involving deuterium, the rotational populations of reactants, together with spin-selection rules, can determine the detailed abundances. Although the review is mainly focused on regions associated with star formation, I also consider chemistry in other interesting astronomical regions—in the early Universe and in the envelopes formed by mass loss during the final stages of stellar evolution.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Interstellar matter comprises the material, gas and dust, between the stars in our and other galaxies.
1.1. Interstellar gas
In broadly defined terms the interstellar gas can be partitioned into several regions all of which are in approximate pressure equilibrium. In this section we concentrate on describing the fundamental physical properties of those regions in which molecules are found. To begin with, though, we note that the general interstellar medium (ISM) contains no photons with energies greater than the ionization potential of hydrogen (13.6 eV) since such photons, which are produced by hot stars, are absorbed entirely in the neighbourhood of each star, ionizing the surrounding gas and forming the so-called Strömgren Sphere. Table 1 presents a list of elemental abundances, ionization potentials and charge state of the elements in the diffuse ISM. Representative conditions for a variety of important cloud types are given in table 2 and the (current) list of interstellar and circumstellar molecules, some 177 firm identifications are given in table 3. In passing, we note that one of the more interesting, and recent, molecules detected is  , the first identification of a noble gas molecule in the interstellar medium. Although
, the first identification of a noble gas molecule in the interstellar medium. Although  Ar is the most abundant isotope on Earth, where it is formed by the beta decay of
Ar is the most abundant isotope on Earth, where it is formed by the beta decay of  K, Barlow et al (2013) detected the
K, Barlow et al (2013) detected the  –0 and 2–1 rotational transitions of
–0 and 2–1 rotational transitions of  in emission towards the Crab Nebula at frequencies of 617.5 and 1234.6 GHz using the Herschel Space Observatory. The detection of this isotope is consistent with its production in the explosive nucleosynthesis that occurred in the supernova explosion that created the Crab Nebula. We see here an example of how observations of molecules can be used to probe physical conditions and, particular, the history of the gas, since much of the chemistry whose products we detect is time-dependent.
in emission towards the Crab Nebula at frequencies of 617.5 and 1234.6 GHz using the Herschel Space Observatory. The detection of this isotope is consistent with its production in the explosive nucleosynthesis that occurred in the supernova explosion that created the Crab Nebula. We see here an example of how observations of molecules can be used to probe physical conditions and, particular, the history of the gas, since much of the chemistry whose products we detect is time-dependent.
Table 1. Solar system abundances relative to hydrogen, ionization potentials and charge state for the most common elements.
| Element | Abundance | I.P. (eV) | Charge state | Element | Abundance | I.P. (eV) | Charge state |
|---|---|---|---|---|---|---|---|
| H | 1 | 13.60 | H | Na | 2.34 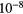 |
5.14 | 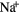 |
| He | 0.1 | 24.59 | He | Mg | 4.17 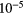 |
7.65 | 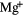 |
| C | 2.9 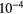 |
11.26 | 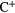 |
Si | 4.07 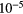 |
8.15 | 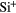 |
| N | 7.9 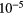 |
14.53 | N | P | 3.47 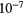 |
10.49 | 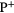 |
| O | 5.8  |
13.62 | O | S | 1.82 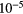 |
10.36 | 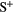 |
| F | 3.39 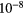 |
17.42 | F | Cl | 2.14 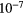 |
12.98 | 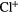 |
Note: In interstellar clouds, these abundances are reduced from cosmic values because all, except H, He and F, have components that are locked up in interstellar grains.
Table 2. Cloud types in the ISM with typical sizes, temperatures, number densities, column densities, visual extinction, fractional ionization and species commonly used to probe physical conditions.
| Cloud type | Size (pc) |
T (K) | n (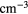 ) ) |
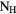 ( (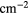 ) ) |
 (mag) (mag) |
f (e) |
Diagnostics |
|---|---|---|---|---|---|---|---|
| Diffuse | 1–3 | 70–100 | 10–100 | 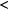  |
 1 1 |
 |
21 cm H line  UV abs UV abs 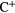 |
| Dark | 1–5 | 8–15 | 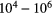 |
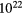 |
10–20 |  |
CO, 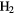 CO HCN, CO HCN, 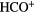 |
| Hot Core | 0.01–0.1 | 100–300 | 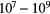 |
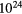 |
5000 | 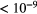 |
CS, CH3OH CH3CN, 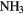 |
| Giant Molecular | 100–500 | 30–70 |  |
 |
— | — | CO, HCN,  |
Table 3. Molecules detected in the gas phase in interstellar and circumstellar clouds (May 2015).
| Size | ||||||
|---|---|---|---|---|---|---|
| 2-atom |  |
CH | 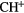 |
NH | OH | 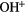 |
| HF | SiH(?) | SH | 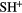 |
HCl | 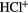 |
|
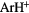 |
 |
CN | 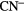 |
CO | 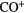 |
|
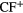 |
SiC | CP | CS | 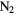 (?) (?) |
NO | |
| SiN | PN | NS | 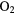 |
AlO | SiO | |
| PO | SO | 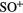 |
FeO(?) | AlF | NaCl | |
| AlCl | SiS | KCl | NO+(?) | |||
| 3-atom | 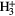 |
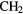 |
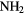 |
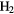 O O |
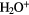 |
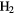 S S |
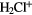 |
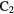 H H |
HCN | HNC | HCO | 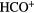 |
|
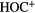 |
HCP | 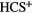 |
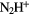 |
HNO | 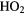 |
|
| AlOH | 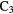 |
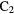 N N |
 O O |
c- |
 P P |
|
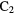 S S |
NaCN | MgNC | MgCN | SiCN | SiNC | |
| KCN | AlNC | FeCN | OCS | 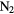 O O |
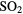 |
|
| SiCSi | ||||||
| 4-atom | 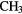 |
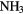 |
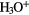 |
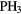 |
 |
 CN CN |
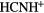 |
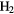 CO CO |
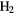 CS CS |
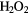 |
c-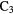 H H |
l-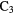 H H |
|
l-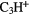 |
HCCN | HNCO | HOCN | HCNO | HMgNC | |
| HNCS | HSCN | 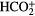 |
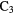 N N |
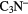 |
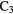 O O |
|
c-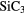 |
 S S |
HCCO | ||||
| 5-atom | 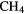 |
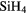 |
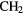 NH NH |
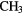 O O |
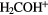 |
c- |
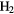 CCC CCC |
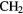 CN CN |
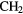 CO CO |
HCOOH | 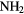 CN CN |
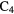 H H |
|
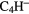 |
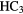 N N |
HCCNC | HNCCC | CNCHO | 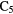 |
|
 |
||||||
| 6-atom | 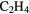 |
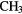 OH OH |
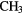 SH SH |
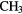 CN CN |
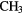 NC NC |
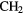 CNH CNH |
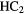 CHO CHO |
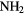 CHO CHO |
 CCCC CCCC |
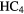 H H |
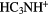 |
c-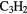 O O |
|
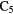 H H |
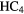 N N |
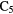 N N |
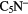 |
 S S |
||
| 7-atom | 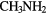 |
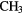 CCH CCH |
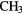 CHO CHO |
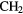 CHCN CHCN |
c-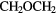 |
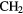 CHOH CHOH |
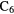 H H |
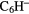 |
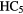 N N |
C2H5SH | |||
| 8-atom | 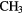 CHNH CHNH |
 CHCHO CHCHO |
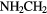 CN CN |
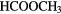 |
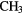 COOH COOH |
 OHCHO OHCHO |
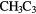 N N |
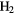 CCCHCN CCCHCN |
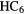 H H |
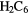 |
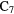 H H |
||
| 9-atom | 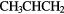 |
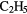 OH OH |
 |
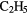 CN CN |
 |
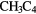 H H |
 H H |
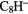 |
 N N |
C2H5SH | |||
| 10-atom | 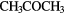 |
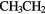 CHO CHO |
(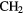 OH)2 OH)2 |
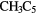 N N |
||
| 11-atom | 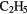 OCHO OCHO |
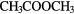 |
 H H |
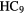 N N |
||
| 12-atom | 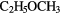 |
 CN CN |
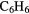 |
|||
| 13-atom | 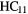 N N |
|||||
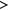 13-atom 13-atom |
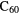 |
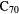 |
||||
As can be seen from table 2, the regions in which molecules are found in the ISM are weakly ionised plasmas with Debye lengths that are typically macroscopic,  10–20 m and plasma parameter,
10–20 m and plasma parameter,  . Despite the relative paucity of charged particles in interstellar clouds, it turns out that exothermic reactions between ions and neutrals control much, though not all, of the basic chemistry that occurs in space.
. Despite the relative paucity of charged particles in interstellar clouds, it turns out that exothermic reactions between ions and neutrals control much, though not all, of the basic chemistry that occurs in space.
1.1.1. Diffuse interstellar clouds.
Diffuse clouds are so called because they are transparent to optical photons and have visual extinctions typically less than 1 magnitude (see the definition of magnitude in section 1.2). They have number densities  100–800
100–800  and line-of-sight depths of a few parsecs. (A parsec (pc) is the distance at which one astronomical unit (AU), roughly an average measure of the Earth–Sun distance, subtends an angle of one arcsec, and is equal to
and line-of-sight depths of a few parsecs. (A parsec (pc) is the distance at which one astronomical unit (AU), roughly an average measure of the Earth–Sun distance, subtends an angle of one arcsec, and is equal to  cm.) Far-ultraviolet photons also penetrate through these clouds, although extinction at FUV wavelengths is several times larger than that in the visible band, and ionize atoms with ionization potentials less 13.6 eV, most importantly in terms of abundance,
cm.) Far-ultraviolet photons also penetrate through these clouds, although extinction at FUV wavelengths is several times larger than that in the visible band, and ionize atoms with ionization potentials less 13.6 eV, most importantly in terms of abundance,  and
and  . The electrons produced by this process and by photoelectric emission from the dust grains heat the gas to kinetic temperatures
. The electrons produced by this process and by photoelectric emission from the dust grains heat the gas to kinetic temperatures  50–100 K with cooling provided by fine-structure transitions in
50–100 K with cooling provided by fine-structure transitions in  ,
,  , O and rotational de-excitations in
, O and rotational de-excitations in  . Since
. Since  is readily ionized by the photon field, the fractional ionization,
is readily ionized by the photon field, the fractional ionization,  is
is  , a large value for an interstellar cloud.
, a large value for an interstellar cloud.
The first molecular species identified in interstellar space, CH,  and CN, were observed through optical absorption spectroscopy around 1940. Since then a number of other, rather simple molecules, including
and CN, were observed through optical absorption spectroscopy around 1940. Since then a number of other, rather simple molecules, including  ,
,  , NH, CO and
, NH, CO and  CO, have been detected through either absorption or, where densities are large enough to provide rotational excitation, millimetre wave emission lines. Fractional abundances of these species are typically in the range
CO, have been detected through either absorption or, where densities are large enough to provide rotational excitation, millimetre wave emission lines. Fractional abundances of these species are typically in the range  .
.
Analysis of the  electronic transitions in many diffuse clouds shows that the fractional abundance of
electronic transitions in many diffuse clouds shows that the fractional abundance of  ,
,  varies from very small values,
varies from very small values,  , to much larger values,
, to much larger values,  0.3, over a very small range of
0.3, over a very small range of  and provides significant insight into the formation and destruction processes of
and provides significant insight into the formation and destruction processes of  . Since the radiative association of two H atoms to form
. Since the radiative association of two H atoms to form  is spin-forbidden, gas-phase routes to its formation must involve ions such as
is spin-forbidden, gas-phase routes to its formation must involve ions such as  and
and  , neither of which are abundant in diffuse clouds and, as a result, can form only insignificant amounts of
, neither of which are abundant in diffuse clouds and, as a result, can form only insignificant amounts of  . The conclusion is that a third body, namely a dust grain, provides the means by which excess energy is removed from the recombination of two hydrogen atoms on its surface. Simple models of this process show that the physical properties of the gas and grains are sufficient to sustain a low fractional abundance, around
. The conclusion is that a third body, namely a dust grain, provides the means by which excess energy is removed from the recombination of two hydrogen atoms on its surface. Simple models of this process show that the physical properties of the gas and grains are sufficient to sustain a low fractional abundance, around  in gas at very small extinctions, on the order of 0.1 magnitudes, irradiated by the average interstellar radiation field.
in gas at very small extinctions, on the order of 0.1 magnitudes, irradiated by the average interstellar radiation field.
The steep rise in  as a function of
as a function of  is due to the fact that
is due to the fact that  is not photodissociated by continuum photons but through the absorption of FUV line photons which excite the molecules into the Lyman and Werner bands, with a significant fraction of the de-excitations,
is not photodissociated by continuum photons but through the absorption of FUV line photons which excite the molecules into the Lyman and Werner bands, with a significant fraction of the de-excitations,  0.15, leading to the vibrational continuum of the ground electronic state. Since these line photons are removed through the absorption process, the intensity of photons at these specific wavelengths decreases rapidly as flux is removed from the photon field much faster than the continuum photons which are predominately absorbed by dust grains—
0.15, leading to the vibrational continuum of the ground electronic state. Since these line photons are removed through the absorption process, the intensity of photons at these specific wavelengths decreases rapidly as flux is removed from the photon field much faster than the continuum photons which are predominately absorbed by dust grains— is said to self-shield—and, as a result, the photodissociation rate of
is said to self-shield—and, as a result, the photodissociation rate of  is several orders of magnitude less than that of other molecules at
is several orders of magnitude less than that of other molecules at  magnitudes.
magnitudes.
Interestingly, Schilke et al (2014) have detected absorption from  and
and  toward several lines of sight through diffuse interstellar material. By comparing with a variety of low density gas tracers, they find that
toward several lines of sight through diffuse interstellar material. By comparing with a variety of low density gas tracers, they find that  is an excellent tracer of atomic hydrogen gas, in particular, it is a unique tracer of gas in which the molecular hydrogen fraction is
is an excellent tracer of atomic hydrogen gas, in particular, it is a unique tracer of gas in which the molecular hydrogen fraction is  . Argon cannot be ionized by the relatively low energy photons in the ISM but by high energy cosmic ray particles. Subsequently
. Argon cannot be ionized by the relatively low energy photons in the ISM but by high energy cosmic ray particles. Subsequently  reacts with
reacts with  to form
to form  . It is destroyed mainly through exothermic proton transfer reaction with
. It is destroyed mainly through exothermic proton transfer reaction with  as its dissociative recombination reaction with electrons is slow.
as its dissociative recombination reaction with electrons is slow.
1.1.2. Dark interstellar clouds.
Dark clouds are so-called because they are opaque to visible and UV radiation and hence 'black out' background star fields. They have number densities 
 . The exclusion of UV photons leads to a number of important consequences for the gas. Photodissociation and photoionization are negligible so that, except for edge effects, both the gas temperature,
. The exclusion of UV photons leads to a number of important consequences for the gas. Photodissociation and photoionization are negligible so that, except for edge effects, both the gas temperature,  10 K, and the fractional ionization,
10 K, and the fractional ionization,  , are much reduced from their values in diffuse clouds.
, are much reduced from their values in diffuse clouds.
The lack of UV photons enables essentially all atomic hydrogen atoms that are converted to molecular form on grain surfaces to survive. The fractional abundance of the residual atomic hydrogen, which is hard to detect in these clouds, is likely to be 
 . Molecules in these clouds are mostly detected through millimetre and submillimetre observations of rotational emission lines which are sensitive down to fractional abundances of
. Molecules in these clouds are mostly detected through millimetre and submillimetre observations of rotational emission lines which are sensitive down to fractional abundances of  depending on the species. To date, over 60 molecules have been detected in these objects and used to show that dark clouds typically have masses
depending on the species. To date, over 60 molecules have been detected in these objects and used to show that dark clouds typically have masses  1–500
1–500  and sizes
and sizes  1–5 pc. High spectral resolution observations show that the line widths in many dark clouds are consistent with thermal motion plus a degree of subsonic turbulence with some clouds showing evidence of infall onto a protostar—a newly forming star. Given the low efficiency of star formation in the Milky Way Galaxy, dark interstellar clouds are likely to form one or two low-mass stars, that is, stars with masses on the order of a solar mass.
1–5 pc. High spectral resolution observations show that the line widths in many dark clouds are consistent with thermal motion plus a degree of subsonic turbulence with some clouds showing evidence of infall onto a protostar—a newly forming star. Given the low efficiency of star formation in the Milky Way Galaxy, dark interstellar clouds are likely to form one or two low-mass stars, that is, stars with masses on the order of a solar mass.
The molecular composition of dark clouds is characterised by two observational results. One is that many of the polyatomic species are carbon-chain molecules, such as cyanopolyynes,  N (
N ( –5), cummulenes
–5), cummulenes  (
( –4), polycarbon sulphides
–4), polycarbon sulphides  S (
S ( –4) and polycarbon monoxides
–4) and polycarbon monoxides  O (
O ( –3),
–3),  N (
N ( ),
),  (
( –8), as well as carbon-chain anions
–8), as well as carbon-chain anions  ,
,  and
and  , perhaps surprising observations given the extremely large abundance of hydrogen relative to carbon in interstellar clouds.
, perhaps surprising observations given the extremely large abundance of hydrogen relative to carbon in interstellar clouds.
The second observation is that many molecules contain enormous enrichments of deuterium, that is the abundance ratio of a deuterated molecule, XD, compared to its hydrogenated analogue, XH, is much larger than that of the cosmic abundance ratio of D to H,  . In these cold, dark clouds, deuterated fractions of up to 0.3 of have been detected (in HDCO). Indeed doubly and triply-deuterated molecules, such as
. In these cold, dark clouds, deuterated fractions of up to 0.3 of have been detected (in HDCO). Indeed doubly and triply-deuterated molecules, such as  CO and
CO and  , have been observed (Lis et al 2002, Parise et al 2006, Roberts and Millar 2007). The abundance ratio of
, have been observed (Lis et al 2002, Parise et al 2006, Roberts and Millar 2007). The abundance ratio of  to
to  is about
is about  , some 11 orders of magnitude greater than the statistical, cosmic ratio, (D/H)
, some 11 orders of magnitude greater than the statistical, cosmic ratio, (D/H) . This enhancement process, called fractionation, is a kinetic effect driven by zero-point energy differences, and allows deuterated species to be very important probes of low temperature physics and chemistry in dark clouds as we will discuss further in section 2.3.
. This enhancement process, called fractionation, is a kinetic effect driven by zero-point energy differences, and allows deuterated species to be very important probes of low temperature physics and chemistry in dark clouds as we will discuss further in section 2.3.
1.1.3. Protoplanetary disks.
The gravitational collapse of interstellar gas in a dark cloud to form a solar-type star proceeds through a protoplanetary disk (PPD), a thin, rotating, flattened structure which acts as an engine that allows mass to accrete on to a central object whilst dissipating angular momentum through turbulent viscosity. A comprehensive model of the physics and chemistry within such a PPD is impossible to make at the present time and here we discuss how one can derive a basic structure for the disk that may be used in chemical kinetic modelling and as a basis of further, more advanced, models. The model is self-consistent in that it adopts hydrostatic equilibrium in the vertical direction and uses local thermal balance to determine the gas temperature. The temperature of the dust grains is calculated separately and, although there are large regions within the disk where the dust and gas are in thermal equilibrium, there are significant regions in which the dust is much cooler than the gas.
Hydrostatic equilibrium in the vertical direction assuming cylindrical,  , coordinates is given by:
, coordinates is given by:
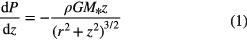
where P is the pressure (P =  kT/
kT/ ), with
), with  , T and
, T and  the density, temperature, and mean molecular mass of the gas and
the density, temperature, and mean molecular mass of the gas and  is the mass of the central protostar. It is useful to write the density distribution in terms of the surface density at a radial distance r,
is the mass of the central protostar. It is useful to write the density distribution in terms of the surface density at a radial distance r,  , defined as
, defined as
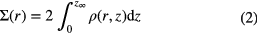
where  represents the surface of the desk and is often taken to be the value of z at which the local density matches that of the ambient molecular material in which the disk is embedded. The radial distribution of matter is found by equating the gravitational energy released by the accreting mass to the thermal heating via viscous dissipation in the disk midplane (Lynden-Bell et al 1974, Pringle 1981) at radius r, so that:
represents the surface of the desk and is often taken to be the value of z at which the local density matches that of the ambient molecular material in which the disk is embedded. The radial distribution of matter is found by equating the gravitational energy released by the accreting mass to the thermal heating via viscous dissipation in the disk midplane (Lynden-Bell et al 1974, Pringle 1981) at radius r, so that:
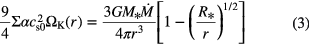
where  and
and  are the sound speed in the disk midplane,
are the sound speed in the disk midplane,  , and Keplerian frequency,
, and Keplerian frequency,  , respectively,
, respectively,  is the stellar radius,
is the stellar radius,  is the mass accretion rate, and
is the mass accretion rate, and  is a viscous parameter related to the turbulent viscosity by
is a viscous parameter related to the turbulent viscosity by  , where H is the scale-height of the disk,
, where H is the scale-height of the disk,  .
.
The radial infall velocity is given by:
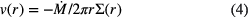
with the accretion timescale,  .
.
In the models described later (section 2.2), we assume  ,
, 
 ,
,  ,
,  ,
,  K and the luminosity
K and the luminosity  , representative of a T Tauri star, and which corresponds to a disk mass of
, representative of a T Tauri star, and which corresponds to a disk mass of  between r = 0.01 and 300 AU (1 AU = average Earth–Sun distance,
between r = 0.01 and 300 AU (1 AU = average Earth–Sun distance,  cm). The accretion velocity is around a few tens of cm
cm). The accretion velocity is around a few tens of cm  so that it takes more than one million years for material to move from the outer edges of the disk, at 300 AU, to the central protostar.
so that it takes more than one million years for material to move from the outer edges of the disk, at 300 AU, to the central protostar.
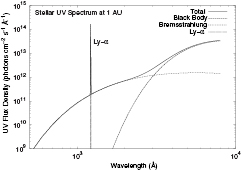
The FUV radiation field, which is important for photoionization, photodissociation and grain heating has four components: blackbody emission at the star's effective temperature,  , intense Lyman alpha emission over a narrow bandwidth, typically 2 Å, optically thin bremsstrahlung emission, and the interstellar radiation field, with a total luminosity of
, intense Lyman alpha emission over a narrow bandwidth, typically 2 Å, optically thin bremsstrahlung emission, and the interstellar radiation field, with a total luminosity of  ergs
ergs  . Figure 1 from Nomura and Millar (2005) shows the radiation field for a typical low-mass protostar. In addition to FUV radiation, ionization is also provided by stellar x-ray photons, cosmic ray particles and radioactive decay, primarily from the decay of
. Figure 1 from Nomura and Millar (2005) shows the radiation field for a typical low-mass protostar. In addition to FUV radiation, ionization is also provided by stellar x-ray photons, cosmic ray particles and radioactive decay, primarily from the decay of  Al to
Al to  Mg—an excess of
Mg—an excess of  Mg found in the Allende meteorite Lee et al (1977) shows that
Mg found in the Allende meteorite Lee et al (1977) shows that  Al was present in the early Solar System and thus is likely to be present in protostellar disks.
Al was present in the early Solar System and thus is likely to be present in protostellar disks.
Figure 1. The ultraviolet spectrum of a typical T Tauri star (from Nomura and Millar (2005) © ESO).
Download figure:
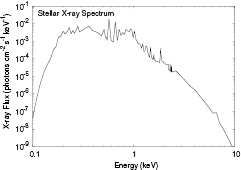
Standard image High-resolution imageThe x-ray flux of T Tauri stars can be significant,  ergs
ergs  , and a typical spectrum, calculated using a two-temperature thermal model with extinction due to ionization of the elements and Compton scattering by hydrogen, is shown in figure 2. Although the flux of x-rays is less than that of FUV radiation, the higher penetration power of x-ray allows them to penetrate to the disk midplane beyond a radius of 10 AU. Cosmic ray particles and radioactive decay provide a low level of ionization in those regions of the disk that are optically thick to the stellar FUV and x-ray radiation.
, and a typical spectrum, calculated using a two-temperature thermal model with extinction due to ionization of the elements and Compton scattering by hydrogen, is shown in figure 2. Although the flux of x-rays is less than that of FUV radiation, the higher penetration power of x-ray allows them to penetrate to the disk midplane beyond a radius of 10 AU. Cosmic ray particles and radioactive decay provide a low level of ionization in those regions of the disk that are optically thick to the stellar FUV and x-ray radiation.
Figure 2. Calculated x-ray spectrum of a T Tauri star taking into account elemental extinction and Compton scattering. The flux is calculated for a T Tauri star at a distance of 56 pc (from Nomura et al (2007) © 2007. The American Astronomical Society.).
Download figure:
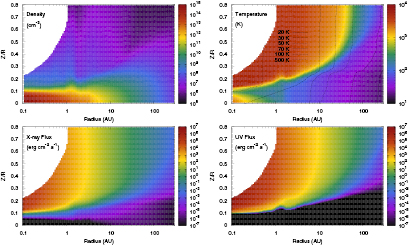
Standard image High-resolution imageSince the gas and grain temperatures have an important influence on the chemistry, for example, controlling freeze-out of the gas to the grains and thermal desorption from the grains, it is important to calculate the thermal balance of both gas and dust as accurately as possible. Woods and Willacy (2009) provide a detailed list of important processes—in essence, energy is input to the PPD through FUV and x-ray radiation and is lost to the system by fine structure transitions of atoms and atomic ions, primarily in the surface layers of the disk, and vibrational and rotational transitions in the molecular gas, as well as through infrared continuum emission from the grains. At high densities the gas and grain temperatures are coupled through collisions but this breaks down in the less dense upper regions of the disk where the gas temperature can reach 4000 K while the grain temperature is typically an order of magnitude less. Figure 3 shows the gas and grain temperatures, density, FUV and x-ray fluxes for the disk model parameters listed above (Walsh et al 2012). One sees that the density ranges over some 10 orders of magnitude and in the hot inner disk, inside 1 AU, can reach values where three-body reactions become important.
Figure 3. Neutral density ( ), temperature (K), x-ray and UV fluxes (erg
), temperature (K), x-ray and UV fluxes (erg 
 ) for a typical low mass T Tauri star (from Walsh et al (2012) © 2012. The American Astronomical Society.).
) for a typical low mass T Tauri star (from Walsh et al (2012) © 2012. The American Astronomical Society.).
Download figure:
Standard image High-resolution imageIt is worth noting at this point that the combination of high density and cold gas and dust throughout a sizeable fraction of the disk will lead to a freeze-out of gas onto the dust grains, forming molecular ice mantles. Such freeze-out occurs on a timescale t(yr)  ), that is, less than
), that is, less than  yr, much less than the typical accretion time-scale mentioned above. Thus we expect that in the disk midplane, far from the protostar, essentially all gas-phase species, with the exception of hydrogen, helium and related species, will have negligible abundance. Thus, the initial state for subsequent chemistry as these dust grains move radially inwards is dominated by the composition of the ice and its desorption kinetics as it is impacted by FUV, x-ray and cosmic ray particles on its journey. Given that these molecular ices may survive for more than
yr, much less than the typical accretion time-scale mentioned above. Thus we expect that in the disk midplane, far from the protostar, essentially all gas-phase species, with the exception of hydrogen, helium and related species, will have negligible abundance. Thus, the initial state for subsequent chemistry as these dust grains move radially inwards is dominated by the composition of the ice and its desorption kinetics as it is impacted by FUV, x-ray and cosmic ray particles on its journey. Given that these molecular ices may survive for more than  year in a disk before removal, it is also important to ascertain whether surface and/or volume chemistry could occur and alter the ice composition before mantle removal.
year in a disk before removal, it is also important to ascertain whether surface and/or volume chemistry could occur and alter the ice composition before mantle removal.
1.1.4. Hot molecular cores.
While PPDs are known to be intimately connected with the formation of low mass stars like our Sun, there is no generally accepted theory for the formation of massive stars, that is, stars with masses greater than about 8– . The basic problem is that if stars form from the gravitational collapse of cloud material, radiation pressure from the ever-growing protostar can halt the infall process before stars with large masses can be made.
. The basic problem is that if stars form from the gravitational collapse of cloud material, radiation pressure from the ever-growing protostar can halt the infall process before stars with large masses can be made.
Observations of the formation of massive stars are surprisingly difficult to make even though their luminosities can be  times larger than their lower-mass counterparts. This difficulty is due to the fact that the time-scale for the onset of nuclear fusion is a strong function of stellar mass, so that massive stars form rapidly, in around
times larger than their lower-mass counterparts. This difficulty is due to the fact that the time-scale for the onset of nuclear fusion is a strong function of stellar mass, so that massive stars form rapidly, in around  yr compared to
yr compared to  yr for a solar-mass star, are very energetic, and disperse their natal material within a few hundred thousand years. As a result, massive stars are more difficult to catch in the star-formation phase and are, in addition, much less numerous than low-mass stars with the result that high-mass, star-forming regions are generally much more distant, by factors of 20–100, than those forming low-mass stars, with a concommitant loss of spatially resolved information.
yr for a solar-mass star, are very energetic, and disperse their natal material within a few hundred thousand years. As a result, massive stars are more difficult to catch in the star-formation phase and are, in addition, much less numerous than low-mass stars with the result that high-mass, star-forming regions are generally much more distant, by factors of 20–100, than those forming low-mass stars, with a concommitant loss of spatially resolved information.
Massive stars do, however, have several advantages for the molecular astrophysicist. Because of their mass they generally form out of more massive interstellar clouds, perhaps up to  , and because of their large luminosities they can heat large masses of dense gas to a very high temperature, at least by interstellar standards,
, and because of their large luminosities they can heat large masses of dense gas to a very high temperature, at least by interstellar standards,  100–300 K. Although this gas is dispersed on timescales of
100–300 K. Although this gas is dispersed on timescales of  years or so, it contains a very rich mix of molecules evaporated from ices, as well as those processed in the gas phase, that are readily observed with radio telescopes.
years or so, it contains a very rich mix of molecules evaporated from ices, as well as those processed in the gas phase, that are readily observed with radio telescopes.
Because it is difficult for some massive stars to heat a significant volume of high-density, molecular gas in competition with efficient cooling through molecular line emission, the sizes of these hot molecular cores (HMCs) are generally small,  0.01–0.1 pc, compared to the larger, colder molecular clouds (or envelopes), which can be 10–100 times larger, in which they are embedded. The molecular composition of HMCs is significantly different to that of cold, dark interstellar clouds and it is this composition that points unambiguously to the role of heterogeneous chemistry. In particular, HMCs contain large abundances of fully hydrogenated molecules such as
0.01–0.1 pc, compared to the larger, colder molecular clouds (or envelopes), which can be 10–100 times larger, in which they are embedded. The molecular composition of HMCs is significantly different to that of cold, dark interstellar clouds and it is this composition that points unambiguously to the role of heterogeneous chemistry. In particular, HMCs contain large abundances of fully hydrogenated molecules such as  ,
,  ,
,  S,
S,  OH,
OH,  OH and so on, with fractional abundances that are enhanced by factors of
OH and so on, with fractional abundances that are enhanced by factors of  over those in dark clouds. In these latter objects, abundances are determined, in the main, by gas-phase chemistry. Although the higher temperatures in HMCs do allow for some gas-phase reactions to proceed more rapidly, this cannot account for all such enhanced abundances. In addition, fractionation of deuterium in HMCs, that is, the abundance ratio of D/H in molecules, is typically 10–100 times the cosmic D/H ratio, although smaller than the enhancements of
over those in dark clouds. In these latter objects, abundances are determined, in the main, by gas-phase chemistry. Although the higher temperatures in HMCs do allow for some gas-phase reactions to proceed more rapidly, this cannot account for all such enhanced abundances. In addition, fractionation of deuterium in HMCs, that is, the abundance ratio of D/H in molecules, is typically 10–100 times the cosmic D/H ratio, although smaller than the enhancements of  seen in dark clouds. As will be discussed in section 1.3, fractionation is not efficient at the temperatures of HMCs and the observed abundances are, rather, indicative of cold chemistry, at
seen in dark clouds. As will be discussed in section 1.3, fractionation is not efficient at the temperatures of HMCs and the observed abundances are, rather, indicative of cold chemistry, at  20–50 K. This, together with the large abundances of hydrogenated molecules, can be explained if the molecules detected in the hot gas phase through rotational line emission are formed as cold ice mantles on dust grains, mantles that are evaporated readily by radiation from a newly-formed, massive star. Simple molecules such as
20–50 K. This, together with the large abundances of hydrogenated molecules, can be explained if the molecules detected in the hot gas phase through rotational line emission are formed as cold ice mantles on dust grains, mantles that are evaporated readily by radiation from a newly-formed, massive star. Simple molecules such as  O and
O and  that evaporate as parents can, of course, take part in subsequent gas-phase chemistry to form daughter molecules, some of which can be quite complex, for example methyl ethyl ether. Since the abundance of such parent and daughter molecules evolve rapidly with time, observations of molecules have the potential to determine time scales in the formation of massive stars as well as to act as effective probes of the cold ice chemistry, a chemistry which may have been occurring for perhaps one million years before the onset of massive star formation returned ice mantles to the gas phase.
that evaporate as parents can, of course, take part in subsequent gas-phase chemistry to form daughter molecules, some of which can be quite complex, for example methyl ethyl ether. Since the abundance of such parent and daughter molecules evolve rapidly with time, observations of molecules have the potential to determine time scales in the formation of massive stars as well as to act as effective probes of the cold ice chemistry, a chemistry which may have been occurring for perhaps one million years before the onset of massive star formation returned ice mantles to the gas phase.
Although relatively small, HMCs are very dense by normal standards for molecular clouds, n( )
) 
 , have masses of a few thousand solar masses and very large column densities, N(
, have masses of a few thousand solar masses and very large column densities, N( )
) 
 , compared to
, compared to 
 in dark clouds, and relatively small electron fractions, on the order of
in dark clouds, and relatively small electron fractions, on the order of  . Thus, although physically small, this combination of density, temperature, mass and large abundances of complex organic molecules means that the sub-millimetre spectra of HMCs are very bright and line rich, indeed observed spectra can be so line rich that line blending causes significant difficulty in identifying specific transitions and molecules.
. Thus, although physically small, this combination of density, temperature, mass and large abundances of complex organic molecules means that the sub-millimetre spectra of HMCs are very bright and line rich, indeed observed spectra can be so line rich that line blending causes significant difficulty in identifying specific transitions and molecules.
1.1.5. Giant molecular clouds.
Although individual molecular clouds do exist, many of the types of environment discussed above can be found within much larger, coherent structures called Giant Molecular Clouds (GMCs). These GMCs can be very large, perhaps several hundred pc in size, and form, with the possible exception of the black hole at the Galactic Centre, the most massive objects in the Milky Way, with typical masses ranging from  . Star formation is a very active process within GMCs, some 3–5% of their mass will eventually end up in new stars, and phenomena associated with star formation—photoionization, stellar winds, supernovae—return significant energy to the molecular gas and can alter appreciably physical and chemical characteristics through, for example, the propagation of hydrodynamic and MHD shocks. Such shock waves can drive a significantly different chemistry in post-shock gas than that described here-to-fore. Typically, shock waves both compress and heat the gas, often to several thousand degrees, and can return heavy atoms, such as Si, to the gas due to sputtering from dust grains. At such high temperatures, reactions of neutral species with H and
. Star formation is a very active process within GMCs, some 3–5% of their mass will eventually end up in new stars, and phenomena associated with star formation—photoionization, stellar winds, supernovae—return significant energy to the molecular gas and can alter appreciably physical and chemical characteristics through, for example, the propagation of hydrodynamic and MHD shocks. Such shock waves can drive a significantly different chemistry in post-shock gas than that described here-to-fore. Typically, shock waves both compress and heat the gas, often to several thousand degrees, and can return heavy atoms, such as Si, to the gas due to sputtering from dust grains. At such high temperatures, reactions of neutral species with H and  become efficient and can lead to abundances much different from colder chemistries. The topic of shock chemistry, important in some specific regions, is discussed briefly in section 2.6.
become efficient and can lead to abundances much different from colder chemistries. The topic of shock chemistry, important in some specific regions, is discussed briefly in section 2.6.
The role of magnetic fields and its coupling to charged particles is particularly important in star formation since they control the ability of gas to undergo gravitational collapse. If the ionization level is high, then collisions between ions and the neutrals couple the neutrals to the magnetic field and create a magnetic pressure which is able to resist collapse. In dense cores, however, the ionization fraction is low, less than  , thereby allowing the neutral gas to decouple from the field and collapse. This process of ambipolar diffusion, which has long been viewed as the standard model for star formation, accounts naturally for the low efficiency of star formation. Since it is a slow process, taking around 10 Myr, molecular clouds are long-lived objects. Whether a particular dense core in a GMC will collapse can be determined by a consideration of its mass-to-flux ratio in comparison to the critical ratio which separates out material which can collapse (supercritical) from that which cannot (subcritical, for example, the molecular cloud envelope).
, thereby allowing the neutral gas to decouple from the field and collapse. This process of ambipolar diffusion, which has long been viewed as the standard model for star formation, accounts naturally for the low efficiency of star formation. Since it is a slow process, taking around 10 Myr, molecular clouds are long-lived objects. Whether a particular dense core in a GMC will collapse can be determined by a consideration of its mass-to-flux ratio in comparison to the critical ratio which separates out material which can collapse (supercritical) from that which cannot (subcritical, for example, the molecular cloud envelope).
This theory of star formation can be probed through measurement of the magnetic field strength through observations of Zeeman splitting in emission lines from molecules such as OH and CN. These give the line-of-sight component,  , of the magnetic field while linear polarization measurements of the thermal continuum emission from dust grains give the field in the plane of the sky. The ionisation fraction cannot be measured directly in clouds but can be estimated from measurements of deuterium fractionation, such as the
, of the magnetic field while linear polarization measurements of the thermal continuum emission from dust grains give the field in the plane of the sky. The ionisation fraction cannot be measured directly in clouds but can be estimated from measurements of deuterium fractionation, such as the  /
/ abundance ratio (see section 2.3). We note though that the use of molecular emission lines, which are sensitive to local densities since they are excited by collisions, means that the measurements necessary to be made may not always sample the same volume of the cloud.
abundance ratio (see section 2.3). We note though that the use of molecular emission lines, which are sensitive to local densities since they are excited by collisions, means that the measurements necessary to be made may not always sample the same volume of the cloud.
Crutcher et al (2009) have performed Zeeman observations of OH toward four molecular cloud cores and their surrounding envelopes and find that the mass-to-flux-ratio in the cores are less than in their corresponding envelopes, the opposite behaviour to that predicted by the ambipolar diffusion model of star formation. This suggests that an alternative theory of star formation may be required. One recent suggestion is that molecular clouds form from the collision of turbulent supersonic flows. Only those, a minority of clouds, which are gravitationally bound collapse rapidly in a free-fall time (section 1.3.3) to form stars; most clouds dissipate thereby keeping the overall efficiency of star formation low, as required. Crutcher (2012) has recently reviewed the role of magnetic fields in molecular clouds.
1.2. Interstellar dust
The presence of dust grains has been known for more than a century and measurements of absorption and scattering of star light from ultraviolet to submillimetre wavelengths have led to a consensus that the grains are non-spherical, sub-micron sized particles composed of silicates and carbonaceous materials. These are most likely formed in the cooling gas following supernovae explosions as well as in the atmospheres of late-type, cool stars which can be either oxygen-rich, with the abundance of O greater than that of C (O/C  ) forming silicates, or carbon-rich, with the abundance of C greater than that of O (O/C
) forming silicates, or carbon-rich, with the abundance of C greater than that of O (O/C  ), forming carbonaceous grains.
), forming carbonaceous grains.
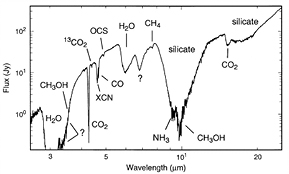
Fitting the observed behaviour of extinction and polarization as a function of wavelength gives a non-unique description of grain size, shape, porosity, and composition although size distributions based on a power-law size distribution of spherical grains or a continuous size distribution of ellipsoids are widely used in the literature. While such dust grains likely remain bare in the general interstellar medium, in cold, denser regions they can be covered by ice mantles. The infrared spectra of such grains, such as that shown in figure 4 (Gibb et al 2000), can be used to identify the most common molecular components of the ice— O, CO,
O, CO,  OH,
OH,  —and their abundances relative to hydrogen. Such observations are sensitive enough to trace molecules down to fractional abundances (relative to hydrogen), of about
—and their abundances relative to hydrogen. Such observations are sensitive enough to trace molecules down to fractional abundances (relative to hydrogen), of about  , that is 1–0.1% of that of water ice, the dominant component of interstellar ices.
, that is 1–0.1% of that of water ice, the dominant component of interstellar ices.
Figure 4. Infrared spectrum of the deeply embedded protostar W33A showing a variety of absorption bands due to solid state molecules frozen on to the surfaces of silicate dust grains (from Gibb et al (2000) © 2000. The American Astronomical Society.).
Download figure:
Standard image High-resolution imageFinally, a number of infrared emission bands have been detected in regions in which grains are irradiated by ultraviolet photons. These bands have been identified with the stretching and bending modes in polycyclic aromatic hydrocarbon (PAHs) and, although no individual PAH has yet been identified in space, the band intensities are consistent with some 10–20% of the total cosmic carbon abundance being in this form. Detailed modelling of the continuous emission seen at 2–10  m indicates that the PAHs must be small,
m indicates that the PAHs must be small,  30–100 carbon atoms. Figure 5 shows an example of the types of PAH molecules that are thought to be present in the interstellar medium together with the 3–20
30–100 carbon atoms. Figure 5 shows an example of the types of PAH molecules that are thought to be present in the interstellar medium together with the 3–20  m spectrum that they give rise to. The origin of these PAHs is unknown but it is likely that they are formed in cool, carbon-rich stars. However, none has been detected in such environments since they lack the source of UV photons that drives the infrared fluorescence. Hence only the most radiation stable are able to survive for periods of
m spectrum that they give rise to. The origin of these PAHs is unknown but it is likely that they are formed in cool, carbon-rich stars. However, none has been detected in such environments since they lack the source of UV photons that drives the infrared fluorescence. Hence only the most radiation stable are able to survive for periods of  years, needed to populate the general interstellar medium (ISM) with dust.
years, needed to populate the general interstellar medium (ISM) with dust.
Figure 5. A selection of the types of PAH molecules though to be present in the interstellar medium. Colour coding: hydrogen (white), carbon (black), nitrogen(red), oxygen (blue), magnesium (cyan).The spectrum is a typical 3–20  m interstellar PAH emission spectrum. Figure courtesy of Christian Boersma and Lou Allamandola.
m interstellar PAH emission spectrum. Figure courtesy of Christian Boersma and Lou Allamandola.
Download figure:
Standard image High-resolution imageAlthough no single PAH has been identified, there is evidence for the presence of specific molecules containing tens of carbon atoms, namely the fullerenes  and
and  . Cami et al (2010) used the Spitzer Space Telescope to detect several infrared emission bands in the 7–19
. Cami et al (2010) used the Spitzer Space Telescope to detect several infrared emission bands in the 7–19  m wavelength range from both molecules in the young planetary nebula Tc 1. Subsequently,
m wavelength range from both molecules in the young planetary nebula Tc 1. Subsequently,  has been detected in a number of different objects including planetary nebulae in both the Milky Way and Large Magellanic Cloud galaxies, in late-type stars, in the transition phase between such stars and planetary nebulae and in some young, star-forming objects. Although seen in a variety of sources, the fullerenes are not common, detected in only around 3% of carbon-rich planetary nebulae searched with Spitzer.
has been detected in a number of different objects including planetary nebulae in both the Milky Way and Large Magellanic Cloud galaxies, in late-type stars, in the transition phase between such stars and planetary nebulae and in some young, star-forming objects. Although seen in a variety of sources, the fullerenes are not common, detected in only around 3% of carbon-rich planetary nebulae searched with Spitzer.
Astronomers use magnitudes to measure brightness with a magnitude difference of five between two objects equal to a brightness ratio of 100. Studies of lines-of-sight to nearby bright stars show that there is a relationship between the column density of hydrogen nuclei,  , and the visual extinction,
, and the visual extinction,  , (absorption + scattering), due to the dust grains implying that the gas and dust are generally well mixed in the ISM. In particular, the ratio is given by
, (absorption + scattering), due to the dust grains implying that the gas and dust are generally well mixed in the ISM. In particular, the ratio is given by  /
/ magnitudes
magnitudes  per H nucleon (Ratchford et al 2009). For atomic hydrogen gas of average density 1
per H nucleon (Ratchford et al 2009). For atomic hydrogen gas of average density 1  over a path length of 1 kpc
over a path length of 1 kpc  cm) this gives rise to 1.6 magnitudes of extinction in the V band (550 mm). In terms of optical depth
cm) this gives rise to 1.6 magnitudes of extinction in the V band (550 mm). In terms of optical depth  .
.
Observational studies of the relationship between the gas and dust have shown that the dust coincides spatially with the gas with a mass ratio of about 1% and an average cross-section per unit volume 
 , where the grains are assumed to be spherical, radius a, and number density
, where the grains are assumed to be spherical, radius a, and number density  per unit volume, and n is the number of hydrogen nuclei per unit volume, with
per unit volume, and n is the number of hydrogen nuclei per unit volume, with  .
.
As well as providing extinction, dust grains also remove heavy elements—C, N, O, Mg, Si, Fe, etc—from the gas phase, so-called elemental depletion, thereby ensuring that not all of an element's cosmic abundance is available for molecule formation. Although the presence of dust reduces the absolute abundances of the elements from which molecules are formed in the gas-phase, grain surfaces also provide an important environment on which chemical species can meet and react. The long periods of time in which an atom, radical or molecule can spend on a dust grain, perhaps in excess of one million years, makes surface chemistry viable even if reaction rates are low due to the very low temperatures, 10–20 K, of the dust particles. Most importantly, grain surfaces provide an extremely efficient environment for the formation of  , a subject to which we return later in this article.
, a subject to which we return later in this article.
The temperature of dust grains in the ISM is determined by the balance between heating, dominated by the absorption of UV photons in regions of low extinction, and collisions with gas and cooling by thermal emission. In regions with densities less than about 
 , collisions are too slow to equilibrate the gas and dust temperatures, and as a result, the grain temperatures can be very low,
, collisions are too slow to equilibrate the gas and dust temperatures, and as a result, the grain temperatures can be very low,  6–10 K in a dark cloud core, to
6–10 K in a dark cloud core, to  15 K in cloud edges, to
15 K in cloud edges, to  20–25 K in diffuse clouds.
20–25 K in diffuse clouds.
1.3. Time scales
At this point, it is worth considering some typical time scales that are relevant in interstellar chemistry.
1.3.1. Chemical time scale.
We can define a chemical time scale from the reaction of neutral species A with a partner X, as:

where  is the abundance (concentration) of X per unit volume and k is the rate coefficient. For ion-neutral reactions,
is the abundance (concentration) of X per unit volume and k is the rate coefficient. For ion-neutral reactions, 

 ; for important, exothermic neutral–neutral reactions,
; for important, exothermic neutral–neutral reactions, 

 . Thus, for a dark cloud, if the colliding partner is
. Thus, for a dark cloud, if the colliding partner is  , the most abundant interstellar molecule, then
, the most abundant interstellar molecule, then  s, around 1 d for reaction with an ion, and about 10–100 d for reaction with a neutral at a density
s, around 1 d for reaction with an ion, and about 10–100 d for reaction with a neutral at a density 
 . Reactions of A with other atoms and molecules occur on a time scale at least 100 times longer. Thus, since collisions with
. Reactions of A with other atoms and molecules occur on a time scale at least 100 times longer. Thus, since collisions with  also de-excite molecules, radiative and collisional decay means that it is generally the case that reactions in the interstellar medium involve species in their ground electronic and vibrational states. In diffuse clouds, photoprocesses are important with
also de-excite molecules, radiative and collisional decay means that it is generally the case that reactions in the interstellar medium involve species in their ground electronic and vibrational states. In diffuse clouds, photoprocesses are important with  yr for the average interstellar radiation field. In dense clouds, ionization is provided by high energy cosmic ray protons on a time scale of
yr for the average interstellar radiation field. In dense clouds, ionization is provided by high energy cosmic ray protons on a time scale of  yr.
yr.
1.3.2. Grain accretion time scale.
Observations of the interstellar extinction of starlight allows one to estimate the surface area of dust grains per unit volume and thus the time-scale for a gas-phase species to collide with an interstellar dust grain. Observations shows that gas and dust in the Milky Way are extremely well mixed, with a fairly constant gas-to-dust mass ratio of 100:1. For the grains responsible for the visual extinction in the interstellar medium, with sizes  0.1
0.1  m, the number of dust grains per unit volume,
m, the number of dust grains per unit volume,  , where n is the density of H nucleons. In diffuse clouds, grains of size
, where n is the density of H nucleons. In diffuse clouds, grains of size  0.01
0.01  m provide extinction in the ultraviolet, larger in magnitude to that in the visible part of the spectrum, and have about 10 times the surface area of the larger grains. In cold dense clouds, these small grains are likely removed by coagulation or conglomeration processes which produce fewer, larger particles with a reduced surface area overall. Using these values we can derive an accretion time scale for species A to freeze-out on the surface of dust as:
m provide extinction in the ultraviolet, larger in magnitude to that in the visible part of the spectrum, and have about 10 times the surface area of the larger grains. In cold dense clouds, these small grains are likely removed by coagulation or conglomeration processes which produce fewer, larger particles with a reduced surface area overall. Using these values we can derive an accretion time scale for species A to freeze-out on the surface of dust as:
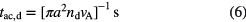
where  is the velocity of A relative to the dust grain, or
is the velocity of A relative to the dust grain, or  yr where n is the number of hydrogen nucleons
yr where n is the number of hydrogen nucleons  . Thus one sees that accretion and ice mantle formation can be a significant, in fact, dominant process in cold, dark clouds and in the cold midplanes of protoplanetary disks.
. Thus one sees that accretion and ice mantle formation can be a significant, in fact, dominant process in cold, dark clouds and in the cold midplanes of protoplanetary disks.
1.3.3. Dynamical time scales.
Since there is a variety of physical processes that drive dynamics in molecular clouds, there is a variety of time scales that can be important. Here we mention only one.
The free-fall time,  , is a crude, miminum measure of the time it takes for a molecular cloud to collapse under the effect of its self-gravity to form a star. If we assume that the cloud is homogeneous with initial hydrogen nucleon density
, is a crude, miminum measure of the time it takes for a molecular cloud to collapse under the effect of its self-gravity to form a star. If we assume that the cloud is homogeneous with initial hydrogen nucleon density 
 , then the free-fall time scale is given by
, then the free-fall time scale is given by  , that is,
, that is,  (in years) is
(in years) is  . This time-scale is, in fact, too short, perhaps by an order of magnitude, when compared to the observed efficiency of star formation, not surprising since its derivation neglects internal pressures, such as turbulence, as well as rotational and magnetic pressures, that oppose gravitational collapse.
. This time-scale is, in fact, too short, perhaps by an order of magnitude, when compared to the observed efficiency of star formation, not surprising since its derivation neglects internal pressures, such as turbulence, as well as rotational and magnetic pressures, that oppose gravitational collapse.
We conclude this section by noting that there are situations in interstellar clouds in which gas-phase chemical reactions, grain accretion, and dynamics will occur on similar times scales and that these lead to a complex, non-linear interplay between physics and chemistry which must be expressed via the specific chemical kinetic and dynamical equations. Once expressed, these equations must be solved in a self-consistent manner in order to understand both chemical evolution and the emission properties of gas in molecular clouds. For the most part, this holistic description is still beyond our reach.
2. Basic chemistry
In this section I shall review the reactions that turn atomic gas into molecular gas in interstellar clouds. We refer to our discussion in section 1.1.1 in which it was pointed out that  forms through the reaction of two hydrogen atoms on the surface of a dust grain. In this section, however, we concentrate on the basic gas-phase chemistry that turns atoms and atomic ions into molecules. We begin with diffuse clouds in which O and N are in neutral form and the other common elements are photoionized by the UV radiation field (see table 1). In all cases where particular rate coefficients are given explicit values, the data are taken from the Rate12 version of the UMIST Database for Astrochemistry at URL www.udfa.net (McElroy et al 2013).
forms through the reaction of two hydrogen atoms on the surface of a dust grain. In this section, however, we concentrate on the basic gas-phase chemistry that turns atoms and atomic ions into molecules. We begin with diffuse clouds in which O and N are in neutral form and the other common elements are photoionized by the UV radiation field (see table 1). In all cases where particular rate coefficients are given explicit values, the data are taken from the Rate12 version of the UMIST Database for Astrochemistry at URL www.udfa.net (McElroy et al 2013).
2.1. Hydride formation
The dominant form of carbon in diffuse clouds,  , does not react with
, does not react with  because of a large endothermicity, but is able form
because of a large endothermicity, but is able form  through an inefficient radiative association:
through an inefficient radiative association:
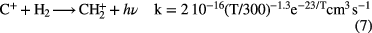
that is, only 1 in  collisions of
collisions of  with
with  lead to
lead to  . In diffuse clouds the most abundant, chemically active species are H,
. In diffuse clouds the most abundant, chemically active species are H,  and
and  .
.  can undergo a number of competing reactions the most important of which are:
can undergo a number of competing reactions the most important of which are:
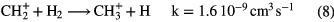
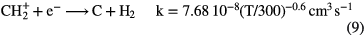
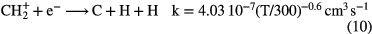
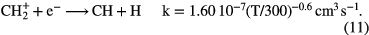
Since the abundance of  is
is  that of
that of  (= the abundance of
(= the abundance of  ),
),  , once formed, reacts quickly with
, once formed, reacts quickly with  to form
to form  . The methyl ion does not react with
. The methyl ion does not react with  as the reaction to form
as the reaction to form  is endoergic but it can undergo radiative association to form
is endoergic but it can undergo radiative association to form  :
:
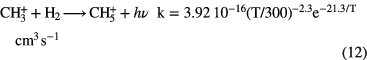
Since this reaction is relatively slow, dissociative recombination of  dominates its loss, producing simple hydride radicals CH and
dominates its loss, producing simple hydride radicals CH and  (Mitchell 1990):
(Mitchell 1990):
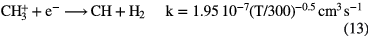
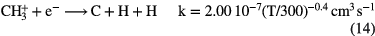
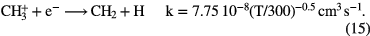
The formation of simple hydride radicals allows for other molecules to form, for example:
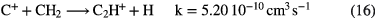
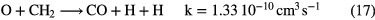
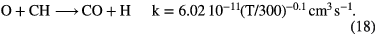
These simple formation routes, when coupled with fast destruction of neutrals by UV photons in diffuse clouds, typically lead to fractional abundances of carbon hydrides and CO around  and
and  , respectively, to that of hydrogen. Thus at most 1% of carbon is processed into molecules in diffuse clouds. Since O is neutral in diffuse clouds and the reaction of O with
, respectively, to that of hydrogen. Thus at most 1% of carbon is processed into molecules in diffuse clouds. Since O is neutral in diffuse clouds and the reaction of O with  is endoergic, oxygen hydrides form by a different process, one that is sensitive to the gas kinetic temperature and to the rate of cosmic-ray ionization.
is endoergic, oxygen hydrides form by a different process, one that is sensitive to the gas kinetic temperature and to the rate of cosmic-ray ionization.
The passage of cosmic rays through interstellar gas leads to ionization, although only that concerned with the most common species H,  and He, plays a significant role (recall that these species cannot be ionized by the UV radiation field). The key to oxygen chemistry is the fact that the ionization potential of O is only slightly larger than that of H, in fact the difference is equivalent to a temperature
and He, plays a significant role (recall that these species cannot be ionized by the UV radiation field). The key to oxygen chemistry is the fact that the ionization potential of O is only slightly larger than that of H, in fact the difference is equivalent to a temperature  K. Thus, if diffuse clouds are warm enough, greater than about 50–60 K, the energy barrier can be overcome, so that:
K. Thus, if diffuse clouds are warm enough, greater than about 50–60 K, the energy barrier can be overcome, so that:
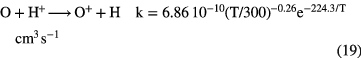
becomes an important loss route for  rather than the slow radiative recombination with electrons.
rather than the slow radiative recombination with electrons.
Once formed,  reacts rapidly with
reacts rapidly with  in a series of exoergic reactions to form
in a series of exoergic reactions to form  which recombines with electrons to form OH and
which recombines with electrons to form OH and  O:
O:

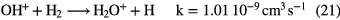
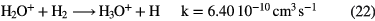
in competition with dissociative recombination of the intermediate ions to form O and OH. The barrier in (19) and photodissociation again restrict abundances—typically OH is more abundant than CH in warm diffuse clouds but the predicted fractional abundance of water is low,  .
.
Like oxygen, nitrogen is neutral in diffuse clouds but there is no easy way of ionizing it in these clouds. Instead, nitrogen chemistry is driven by a series of neutral-neutral reactions such as:
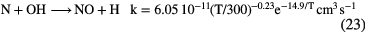
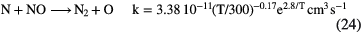
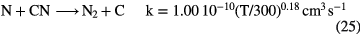
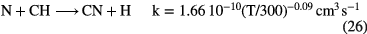
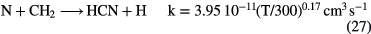
and the abundance of N-bearing molecules is generally small, with CN and HCN having fractional abundances  . This combination of inefficient initiation of the chemistry, rapid dissociative recombination with electrons, due to high fractional ionization, and rapid photodissociation, means that molecules other than
. This combination of inefficient initiation of the chemistry, rapid dissociative recombination with electrons, due to high fractional ionization, and rapid photodissociation, means that molecules other than  take up less than 1% of the elemental abundances and generally contain rather few, usually less than four, atoms.
take up less than 1% of the elemental abundances and generally contain rather few, usually less than four, atoms.
In cold, dark clouds, however, molecule formation can be very efficient, in major part due to the lack of UV photons but also to the presence of a key interstellar ion  . In these objects all elements, with the exception of hydrogen which is largely in the form of
. In these objects all elements, with the exception of hydrogen which is largely in the form of  , begin as neutral atoms. The lack of UV photons has another important consequence for chemistry, namely that, only cosmic ray protons provide ionization. The much smaller ionization rates associated with this process, coupled with rapid dissociative recombination of molecular ions, leads to the result that the fractional ionization in dark clouds is low,
, begin as neutral atoms. The lack of UV photons has another important consequence for chemistry, namely that, only cosmic ray protons provide ionization. The much smaller ionization rates associated with this process, coupled with rapid dissociative recombination of molecular ions, leads to the result that the fractional ionization in dark clouds is low,  compared to
compared to  in diffuse clouds.
in diffuse clouds.
In dark clouds, the basic driver for chemical synthesis of the hydrides, and indeed other molecules, is cosmic-ray ionization of the two most abundant gas-phase species,  and He (the ionization of atomic hydrogen is not important here since its abundance is low):
and He (the ionization of atomic hydrogen is not important here since its abundance is low):
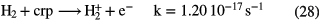
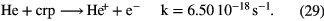
The rates for cosmic-ray ionization quoted above are theoretical estimates based on assumptions about the interstellar flux of low-energy cosmic rays. Since these cosmic rays do not reach Earth we have only a poor handle on the ionization rates. Fortunately, spectrometers on large telescopes are now able to measure the absorption bands of  in the near infrared and can lead to a more accurate estimate of the rate. Consider the case of
in the near infrared and can lead to a more accurate estimate of the rate. Consider the case of  in diffuse clouds where it is formed by cosmic ray ionization of
in diffuse clouds where it is formed by cosmic ray ionization of  and destroyed by dissociative recombination with electrons with rate coefficient
and destroyed by dissociative recombination with electrons with rate coefficient 

 . Assuming steady-state, a good approximation in these clouds, the ionization rate of
. Assuming steady-state, a good approximation in these clouds, the ionization rate of  ,
,  , is given by:
, is given by:
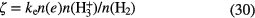
As stated in section 1.1.1, the electron abundance can be taken to be that of  , so that all quantities on the right-hand side can be measured either astronomically or in the laboratory. Indriolo and McCall (2012) have used this approach to determine
, so that all quantities on the right-hand side can be measured either astronomically or in the laboratory. Indriolo and McCall (2012) have used this approach to determine  toward over 20 lines of sight and derive a mean value of
toward over 20 lines of sight and derive a mean value of 
 , somewhat larger than the value in (28) above. The variation of
, somewhat larger than the value in (28) above. The variation of  toward different sight lines and a decrease in its value as the column density of hydrogen increases towards those typical of dense clouds indicates that magnetic fields may shield dense gas from low-energy cosmic ray protons.
toward different sight lines and a decrease in its value as the column density of hydrogen increases towards those typical of dense clouds indicates that magnetic fields may shield dense gas from low-energy cosmic ray protons.
 is not reactive with
is not reactive with  (if it was, the
(if it was, the  abundance in molecular clouds would be significantly less) whilst the
abundance in molecular clouds would be significantly less) whilst the  reacts immediately with
reacts immediately with  , that is within a day or so depending on density, to form
, that is within a day or so depending on density, to form  . Because of its low proton affinity (PA)—of all the abundant interstellar species only
. Because of its low proton affinity (PA)—of all the abundant interstellar species only  and N have PAs less than that of
and N have PAs less than that of  reacts with atoms. Thus carbon–hydrogen bonds are made via:
reacts with atoms. Thus carbon–hydrogen bonds are made via:
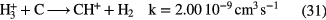
with further exoergic hydrogen abstraction reactions with  converting
converting  to
to  . As in diffuse clouds,
. As in diffuse clouds,  does not react with
does not react with  to form
to form  but instead undergoes radiative association to form
but instead undergoes radiative association to form  . The low electron abundance in dense clouds, however, means that dissociative recombination is much less important a loss for ions that react, even slowly with
. The low electron abundance in dense clouds, however, means that dissociative recombination is much less important a loss for ions that react, even slowly with  , that is, a reaction of an ion and
, that is, a reaction of an ion and  with a rate coefficient larger than
with a rate coefficient larger than 

 would ensure that dissociative recombination, with a rate coefficient on the order of
would ensure that dissociative recombination, with a rate coefficient on the order of 

 , is unimportant.
, is unimportant.
In a similar fashion to C atoms, reaction of O with  produces
produces  which reacts with
which reacts with  in a rapid sequence of reactions to form
in a rapid sequence of reactions to form  . Since neither
. Since neither  nor
nor  react with
react with  , dissociative recombination is now an important loss mechanism, producing reactive radicals such as
, dissociative recombination is now an important loss mechanism, producing reactive radicals such as  ,
,  , O, and OH as well as the closed shell molecules,
, O, and OH as well as the closed shell molecules,  and
and  O.
O.
Reaction schemes similar to these occur for other elements, for example sulfur and chlorine. For nitrogen, however, the formation of N-H bonds in the gas phase is much less efficient because N cannot accept a proton from  and cannot react with
and cannot react with  at very low temperatures. Ammonia,
at very low temperatures. Ammonia,  , is made in an sequence of reactions that require that
, is made in an sequence of reactions that require that  is already present in the gas, via (24) and (25) above. Collisions of
is already present in the gas, via (24) and (25) above. Collisions of  with
with  produce kinetically excited
produce kinetically excited  and N atoms, with the
and N atoms, with the  ions having sufficient energy to overcome the small activation energy barrier of its reaction with ground state
ions having sufficient energy to overcome the small activation energy barrier of its reaction with ground state  ,
,  K:
K:

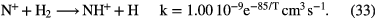
Once  forms, it undergoes a series of hydrogen abstraction reactions with
forms, it undergoes a series of hydrogen abstraction reactions with  to form
to form  , followed by dissociative recombination to form
, followed by dissociative recombination to form  and other N–H bearing radicals. A consequence of this very indirect synthesis is that ammonia abundances are relatively small,
and other N–H bearing radicals. A consequence of this very indirect synthesis is that ammonia abundances are relatively small,  , in molecular clouds, at least those in which gas-phase chemistry dominates.
, in molecular clouds, at least those in which gas-phase chemistry dominates.
Once small radicals and quasi-stable ions, i.e. those which do not, or at most only slowly, react with  , form, molecule formation can proliferate. Thus:
, form, molecule formation can proliferate. Thus:
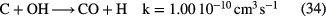
forms CO which is the most abundant molecule in the Universe after  and which takes up essentially all of the available carbon in many environments (recall that in the ISM the elemental abundance of oxygen is greater than that of carbon, table 1). The hydroxyl radical is reactive with a range of atoms and radicals at low temperatures and drives the formation of simple oxides and dioxides:
and which takes up essentially all of the available carbon in many environments (recall that in the ISM the elemental abundance of oxygen is greater than that of carbon, table 1). The hydroxyl radical is reactive with a range of atoms and radicals at low temperatures and drives the formation of simple oxides and dioxides:
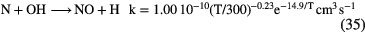
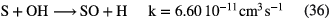
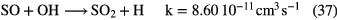
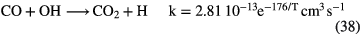
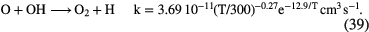
 because of its large ionization potential, generally fragments neutral molecules upon collision, thus generating a population of important reactive ions and radicals, e.g.:
because of its large ionization potential, generally fragments neutral molecules upon collision, thus generating a population of important reactive ions and radicals, e.g.:
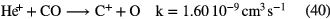
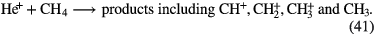
2.2. The origin of complexity
The growth of hydrocarbon molecules can occur one C atom at a time through reactions of C and  , for example:
, for example:
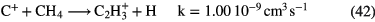
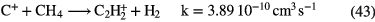
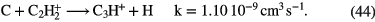
 does not react with
does not react with  while
while  undergoes a slow radiative association:
undergoes a slow radiative association:
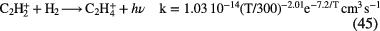
so that the  -bearing cations recombine with electrons to form
-bearing cations recombine with electrons to form  ,
,  H and
H and  . This also enables hydrocarbons to grow in units of
. This also enables hydrocarbons to grow in units of  , for example:
, for example:
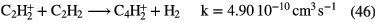
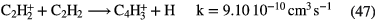
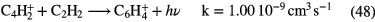
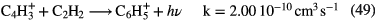
where the rate coefficients for the last two reactions are calculated values and are valid only for the 10–50 K temperature range.
In general hydrocarbon ions of the type  are unreactive with
are unreactive with  for
for  and
and  2 so that, following dissociative recombination, linear carbon chains such as
2 so that, following dissociative recombination, linear carbon chains such as  and
and  H are predicted, and observed, to be abundant in cold clouds. Reactions of radicals and atoms with hydrocarbons create a variety of heterogeneous species:
H are predicted, and observed, to be abundant in cold clouds. Reactions of radicals and atoms with hydrocarbons create a variety of heterogeneous species:
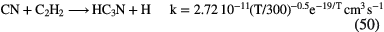
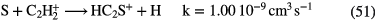
which via analogous reactions involving larger hydrocarbons, produce the cyanopolyynes  N (
N ( –5) and
–5) and  S (
S ( –4). Hydrocarbon chains terminated with O, N and S are also observed in these clouds, again the most important hydrocarbon ions in the synthetic chemistry are quasi-stable with respect to reaction with
–4). Hydrocarbon chains terminated with O, N and S are also observed in these clouds, again the most important hydrocarbon ions in the synthetic chemistry are quasi-stable with respect to reaction with  since these will, in the main, have the largest abundances.
since these will, in the main, have the largest abundances.
The quasi-stable ions play a significant role in the fractionation of deuterium (discussed in section 2.3) and in the production of large, complex organic molecules via radiative association (recall that in most molecular regions the densities are too low for collisional stabilisation). In this respect the methyl ion dominates via reactions such as:
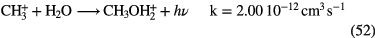

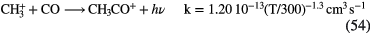
where the rate coefficients are valid over the temperature range 10–300 K. The ions, following dissociative recombination, lead to the observed interstellar molecules methanol,  OH, methyl cyanide,
OH, methyl cyanide,  CN, and ketene,
CN, and ketene,  CO. In some cases, however, most importantly methanol, the branching channel to the desired neutral molecule, is too small to explain its abundance. The important conclusion, nevertheless, is that radiative association is an important process in the efficient synthesis of large organic molecules in space. Indeed, it is difficult to find any efficient gas-phase mechanism that can form
CO. In some cases, however, most importantly methanol, the branching channel to the desired neutral molecule, is too small to explain its abundance. The important conclusion, nevertheless, is that radiative association is an important process in the efficient synthesis of large organic molecules in space. Indeed, it is difficult to find any efficient gas-phase mechanism that can form  OH at its observed abundance in interstellar clouds.
OH at its observed abundance in interstellar clouds.
Although the above discussion has concentrated on the synthetic power of ion-neutral reactions, those between neutral species can also be important, although only where they are exoergic and do not possess activation energy barriers, for example, the reactions:
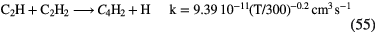
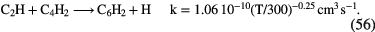
Figure 6 shows the chemical evolution of selected species as a function of time for a cold (10 K), dark cloud model at a density n(
 when the freeze out of molecules on to the grain surface is ignored. We note that, for molecules possessing a permanent electric dipole moment, rotational transitions are readily observable with radio and sub-millimetre telescopes above fractional abundances of about
when the freeze out of molecules on to the grain surface is ignored. We note that, for molecules possessing a permanent electric dipole moment, rotational transitions are readily observable with radio and sub-millimetre telescopes above fractional abundances of about  . One sees a number of general trends in this plot. The first is that, at this density, hydrocarbons form fairly rapidly, on a time scale of about 1000 yr due to a rapid conversion of
. One sees a number of general trends in this plot. The first is that, at this density, hydrocarbons form fairly rapidly, on a time scale of about 1000 yr due to a rapid conversion of  , the initial form of carbon, driven by reaction (7). As the chemistry evolves in this environment, the gas neutralises due to dissociative recombination with electrons and the
, the initial form of carbon, driven by reaction (7). As the chemistry evolves in this environment, the gas neutralises due to dissociative recombination with electrons and the  is converted into C atoms, which also drive hydrocarbon formation through reaction (31). The abundance of
is converted into C atoms, which also drive hydrocarbon formation through reaction (31). The abundance of  is closely related to that of
is closely related to that of  through reactions (46) and (47) and through the neutral-neutral reactions (55) and (56) and its time evolution mimics that of
through reactions (46) and (47) and through the neutral-neutral reactions (55) and (56) and its time evolution mimics that of  .
.
Figure 6. The time-dependent evolution of some hydrcarbons and cyanopolyynes in a cold, dark cloud environment.
Download figure:
Standard image High-resolution imageThe cyanopolyynes form via ion-neutral and neutral-reactions, although the latter tend to dominate. Reactions between CN and polyacetylenes, similar to reaction (50), are particularly efficient; their importance in interstellar clouds has been confirmed by measurements of the various  C isotopologues in
C isotopologues in  N and
N and  N which show that, unlike its other carbon atoms, the carbon atom bonded to nitrogen in the cyanopolyynes has the same isotopic ratio as that in CN. The cyanopolyynes do not show the initial abundance peak of the hydrocarbons at 1000 yr due to the difficulty in forming N-H bonds as discussed above. In this case, one sees that their abundances track those of HCN, with peak abundances reached on time scales of a few times
N which show that, unlike its other carbon atoms, the carbon atom bonded to nitrogen in the cyanopolyynes has the same isotopic ratio as that in CN. The cyanopolyynes do not show the initial abundance peak of the hydrocarbons at 1000 yr due to the difficulty in forming N-H bonds as discussed above. In this case, one sees that their abundances track those of HCN, with peak abundances reached on time scales of a few times  yr. On longer time scales, essentially all carbon is processed into (unreactive) CO and the abundances of atoms, radicals and molecules fall to steady-state values that are much less than their peak values.
yr. On longer time scales, essentially all carbon is processed into (unreactive) CO and the abundances of atoms, radicals and molecules fall to steady-state values that are much less than their peak values.
While the above discussion is useful in identifying the main linkages between species and the relevant chemical time scales, it ignores one important process in cold clouds, namely the fact that all species heavier than hydrogen and helium are expected to freeze out onto the surfaces of the dust grains, as discussed in section 1.3.2. Figure 7 shows the effects of accretion onto grains. In this case, the abundance of HCN, for example, is reduced at times less than  yr and all molecules are removed from the gas around one million years. One consequence of this is that, under the assumption of normal grain properties, cold molecular clouds at high density should be devoid of molecules if they are older than one million years, unless there are non-thermal mechanisms, such as interactions with UV photons or cosmic rays, at work to return ice mantles to the gas.
yr and all molecules are removed from the gas around one million years. One consequence of this is that, under the assumption of normal grain properties, cold molecular clouds at high density should be devoid of molecules if they are older than one million years, unless there are non-thermal mechanisms, such as interactions with UV photons or cosmic rays, at work to return ice mantles to the gas.
Figure 7. The time-dependent evolution of some hydrocarbons and cyanopolyynes in a cold, dark cloud environment when species are allowed to freeze onto grain surfaces.
Download figure:
Standard image High-resolution imageThe most complex interaction of physics and chemistry occurs in protoplanetary disks (section 1.1.3) and is currently a topic of intense research, both observationally and theoretically. Henning and Semenov (2013) give a thorough review of the observations and the range of chemical kinetic models currently in use by the community. In disk systems, photons are generated by three distinct processes: ultraviolet radiation from the central protostar, which includes an excess of Lyman- emission, and which can be time-dependent in nature due to stellar flares; free-free, or
emission, and which can be time-dependent in nature due to stellar flares; free-free, or  radiation; and the external interstellar radiation field which can be enhanced above its average value if the PPD sits within an area of active star formation, as happens, for example, in the Orion Nebula. In addition to UV photons, young protostars also emit copious amounts of x-ray photons which have a greater penetrating power than UV and can ionize material deep in the disk. The decay of radionuclides in the disk provides a lower limit to the degree of ionization in the midplane while external cosmic rays can also ionize material deep into the disk, provided they are not shielded by magnetic fields. Figure 8 shows the typical abundance distributions of several molecules, calculated under two approximations, one—labelled 'old'—in which the wavelength-dependence of the photo-cross-sections are ignored. Here, photo-rates are calculated at each of the 12 000 grid points by scaling the interstellar photo-rates by the dust extinction at each point, the other—labelled 'new'—in which the photo-rates are calculated at each point by integrating the wavelength-dependent product of the cross-section and the UV flux.
radiation; and the external interstellar radiation field which can be enhanced above its average value if the PPD sits within an area of active star formation, as happens, for example, in the Orion Nebula. In addition to UV photons, young protostars also emit copious amounts of x-ray photons which have a greater penetrating power than UV and can ionize material deep in the disk. The decay of radionuclides in the disk provides a lower limit to the degree of ionization in the midplane while external cosmic rays can also ionize material deep into the disk, provided they are not shielded by magnetic fields. Figure 8 shows the typical abundance distributions of several molecules, calculated under two approximations, one—labelled 'old'—in which the wavelength-dependence of the photo-cross-sections are ignored. Here, photo-rates are calculated at each of the 12 000 grid points by scaling the interstellar photo-rates by the dust extinction at each point, the other—labelled 'new'—in which the photo-rates are calculated at each point by integrating the wavelength-dependent product of the cross-section and the UV flux.
Figure 8. The spatial distribution of some simple molecular species are shown for the typical physical conditions in a disk surrounding a low mass, T Tauri star. These abundances were calculated using the physical model of the disk described in section 1.1.3 (from Walsh et al (2012) © 2012. The American Astronomical Society.).
Download figure:
Standard image High-resolution imageOne sees from these plots that, in general terms, the disk separates into three major chemical regions. The first is the disk surface where the intense radiation fields and high temperatures cause photodissociation and photoionization of the gas. The surface is therefore rich in atomic ions and devoid of molecules. The second region is the midplane layer of the disk in which gas-phase abundances vary dramatically depending on radial distance from the central protostar. Far from the star, typically at distances greater than 10 AU, the combination of high density and low temperatures results in freeze-out of neutral species on to the ice mantles of dust grains. As material flows in towards the central star, the dust grains warm up to a radius where, depending on the particular binding energy of the neutral species in the ice, molecules are thermally desorbed to the gas. Thus, water, with binding energy of around 4800 K, does not evaporate until a radius of just over 1 AU ( 1.5
1.5  cm), and has a very large gas-phase abundance inside this radius, whereas
cm), and has a very large gas-phase abundance inside this radius, whereas  , with a binding energy of 2990 K, desorbs at a radius of about 15 AU. It has a large abundance in the midplane between 1 and 10 AU but is destroyed by photons and chemical reactions at smaller radii. The third region evident in these plots is the 'hot molecular layer', seen most clearly in the distributions of
, with a binding energy of 2990 K, desorbs at a radius of about 15 AU. It has a large abundance in the midplane between 1 and 10 AU but is destroyed by photons and chemical reactions at smaller radii. The third region evident in these plots is the 'hot molecular layer', seen most clearly in the distributions of  O,
O,  and
and  . This region has a lower boundary where molecules are in solid rather than gaseous form and an upper boundary where they are destroyed by a combination of photons and high-temperature chemistry.
. This region has a lower boundary where molecules are in solid rather than gaseous form and an upper boundary where they are destroyed by a combination of photons and high-temperature chemistry.
The distributions, however, are also dependent on on poorly-understood physical and chemical parameters, as is evident from figure 8 when one compares the two different approaches to calculation of the photo-rates. While in principle, there is a 'correct' way in which to calculate these, the lack of detailed cross-sections makes this impossible in practice so that approximations need to be made; such approximations can lead to very different distributions. It should also be noted that the intensity and spectral dependence of the UV flux is a complex function of the protostar's mass and age and may be subject to outbursts in intensity due to episodic rather than steady mass accretion. Fogel et al (2011), for example, looked at the particular influence of Ly radiation which in some T Tauri stars can account for 75% of the UV flux. Its influence on molecular distributions depends both on how it is scattered to deep layers in the disk and to the particular values of molecular photodissociation cross-sections at 121.6 nm.
radiation which in some T Tauri stars can account for 75% of the UV flux. Its influence on molecular distributions depends both on how it is scattered to deep layers in the disk and to the particular values of molecular photodissociation cross-sections at 121.6 nm.
Another area of uncertainty in the chemical modelling of PPDs is the rate at which molecular hydrogen forms on dust grains at low temperatures. The overall formation rate must depend on a number of factors, including sticking coefficients, the nature of adsorption sites and their binding energies, scanning rates of H atoms across the surface, and global grain parameters including size, shape, composition, porosity, etc.
Figure 9 shows the effects of two different approaches for calculating the formation rate of  in molecular clouds. Model A is the 'traditional'—and fairly simplistic—model of Hollenbach and Salpeter (1971) whereas Model B follows the approach taken by Cazaux and Tielens (2002) which considers a range of grain types and properties. This figure, which plots the emergent LTE (local thermodynamic equilibrium) line and continuum spectrum for a face-on disk, shows that emission is very sensitive to the particular choice of the
in molecular clouds. Model A is the 'traditional'—and fairly simplistic—model of Hollenbach and Salpeter (1971) whereas Model B follows the approach taken by Cazaux and Tielens (2002) which considers a range of grain types and properties. This figure, which plots the emergent LTE (local thermodynamic equilibrium) line and continuum spectrum for a face-on disk, shows that emission is very sensitive to the particular choice of the  formation rate, indeed much more sensitive than would appear from consideration, for example, of the vertical column densities in the disk. Model A, in particular, shows weak absorption features in
formation rate, indeed much more sensitive than would appear from consideration, for example, of the vertical column densities in the disk. Model A, in particular, shows weak absorption features in  O and
O and  longward of 10
longward of 10  m but strong and broad emission lines of
m but strong and broad emission lines of  O shortward of this wavelength. Absorption lines are due to the fact that both species are abundant near the disk midplane but less abundant in warmer gas closer to the disk surface. The different treatment of
O shortward of this wavelength. Absorption lines are due to the fact that both species are abundant near the disk midplane but less abundant in warmer gas closer to the disk surface. The different treatment of  formation in Model B allows for local differences in the H/
formation in Model B allows for local differences in the H/ ratio to propagate effectively via chemical reactions to other species, in particular to more effective formation of
ratio to propagate effectively via chemical reactions to other species, in particular to more effective formation of  O and OH in warm gas in the disk between 3 and 20 AU. The lines predicted by Model B are stronger than those observed, although this may not necessarily imply that Model B is inappropriate given the fact that the LTE approximation has been used. Non-LTE approaches to radiative transfer generally show that LTE over-estimates the emission by factors of a few.
O and OH in warm gas in the disk between 3 and 20 AU. The lines predicted by Model B are stronger than those observed, although this may not necessarily imply that Model B is inappropriate given the fact that the LTE approximation has been used. Non-LTE approaches to radiative transfer generally show that LTE over-estimates the emission by factors of a few.
Figure 9. The infrared spectra of a variety of molecules are shown, together with the thermal emission of dust grains, for a face-on disk. Models A and B represent two different approaches to the formation of  on dust grains and can lead to widely different absorption and emission spectra (from Heinzeller et al (2011) © 2011. The American Astronomical Society.).
on dust grains and can lead to widely different absorption and emission spectra (from Heinzeller et al (2011) © 2011. The American Astronomical Society.).
Download figure:
Standard image High-resolution imageOne important issue often overlooked in studies of the chemistry of PPDs is that of dynamics. Disks are, by definition, dynamical objects. In addition to rotation, material undergoes radial advection and there is evidence, both from our Solar System and elsewhere, that turbulent motions occur in both the radial and vertical directions. In the early stages of star formation, the forming protostar tends to drive bipolar jets perpendicular to the disks which can both affect chemistry, for example through the shock disruption of dust grains, and alter the radiative transfer of stellar radiation by creating cavities in the surrounding gas. The transfer of stellar radiation is also affected by the growth and settling of dust grains and by the creation of holes and rings in the disk through the interaction of newly formed planets with the gas. The global structure and hence chemistry can also be affected by events such as episodic accretion, that is where the accretion rate of matter on to the star is not steady, by stellar outbursts, in which the radiation and particle fields can be enhanced by many orders of magnitude for short periods of time, and by the photo-evaporation of the disk gas by large UV fluxes from the central or nearby stars, a so-called disk wind.
The incorporation of such dynamics into chemical kinetic models is difficult since the dynamical time scales can be comparable to, or shorter than, the chemical time scales. This leads to increased numerical instabilities and results in models which use a much reduced spatial grid. For example, Heinzeller et al (2011) have investigated the effects of radial advection, vertical turbulence and disk winds on the abundance distributions in a T Tauri disk. They made a simplifying assumption, given that molecular abundances determine the thermal balance in the disk, that while dynamics influenced the chemical distributions, the new distributions did not feed back into a new physical structure for the disk. They were then limited to a grid of around 400 cells compared to over 12 000 used in the chemical model of a steady-state disk (Walsh et al 2012). Figure 10 shows the emergent infrared spectra for three of their models: Model A, discussed above; ACR, in which radial advection is included; and MIX, in which vertical turbulence is included. Furuya et al (2013) extended this model to investigate deuterium chemistry in turbulent disks although, as in Heinzeller et al (2011), on a relatively coarse grid, around 2000 points in total.
Figure 10. The infrared spectra of a variety of molecules are shown, together with the thermal emission of dust grains, for a face-on disk. Models A is for the steady-state disk, ACR includes the effects of radial advection and MIX incorporates vertical turbulence (from Heinzeller et al (2011) © 2011. The American Astronomical Society.).
Download figure:
Standard image High-resolution imageSeveral authors have considered models which combine chemistry and radiation (magneto)hydrodynamics in a post-processing manner, that is where physical conditions are derived in a flow as a function of time and then chemical kinetic models are evolved using these conditions. Visser et al (2011) considered trajectories of material falling on to a PPD in a 2D, semi-analytic approach. More recently, Drozdovskaya et al (2014) extended this approach to calculate the chemistry along several hundred streamlines as material flowed on to and in to a disk from a molecular cloud envelope. While this particular paper considered the PPD as the end point of the calculation, other authors have used much more detailed 3D radiation magnetohydrodynamic codes to derive physical conditions. One of the most impressive of these models is that by Hincelin et al (2013) who followed the collapse of a rotating cloud with an embedded magnetic field up to the point where the first hydrostatic core formed. Even with the simplification of calculating the chemistry once the physical parameters had been determined, they required some  h of computing time to calculate chemistry along the trajectories of some
h of computing time to calculate chemistry along the trajectories of some  particles. The chemical properties of these first cores are important since it is thought that it is this material which goes on to form the PPD. Finally, we mention the work of Ilee et al (2011) who considered the chemistry in a relatively massive, rotating PPD. Such disks, which form around stars with masses greater than the Sun, are subject to gravitational instabilities. These lead to the formation of spiral arms and shock waves which cause molecular abundances to vary on very short temporal and spatial scales. Again, the complexity of the physics allows the chemistry to be followed only for short times, less than 1000 yr in this case.
particles. The chemical properties of these first cores are important since it is thought that it is this material which goes on to form the PPD. Finally, we mention the work of Ilee et al (2011) who considered the chemistry in a relatively massive, rotating PPD. Such disks, which form around stars with masses greater than the Sun, are subject to gravitational instabilities. These lead to the formation of spiral arms and shock waves which cause molecular abundances to vary on very short temporal and spatial scales. Again, the complexity of the physics allows the chemistry to be followed only for short times, less than 1000 yr in this case.
An interesting question that arises in the study of our Solar System is whether primitive bodies such as comets and meteorites might contain prebiotic molecules thereby enhancing the possibility that the development of life on Earth was enhanced by exogenous delivery. If so, then it is of interest to see whether complex organic molecules, or COMs, can form in abundance in PPDs and perhaps deliver prebiotic material to those exoplanets forming in the habitable zone of protostars.
Walsh et al (2014) have investigated this through incorporating a large gas-phase chemistry with an extensive set of surface reactions from Laas et al (2011) into their standard disk model with the chemistry of COMs such as propyne, acetaldehyde, methylamine, methyl formate, acetic acid, ethanol, dimethyl ether and acetone included. Figure 11 presents vertical column densities of several COMs as a function of disk radius and shows that COMs are much more abundant in ices than in the gas phase, reflecting the fact that material near the cold and dense midplane dominates the column density. Although it is not entirely clear from this figure, the ice column densities fall off sharply inside a few AU as ice mantles get evaporated with a corresponding rise in the gas-phase column densities. The latter are, however, not as large as the former due to the destruction of gas-phase COMs in the inner disk through a combination of photoreactions and high-temperature chemistry. Walsh et al used their calculations to determine disk-integrated line spectra in order to determine the observability of COMs with the new ALMA interferometer but found that their detection in even the nearest PPDs will be challenging, even when the full ALMA configuration becomes available.
Figure 11. Column densities ( ) as a function of radius for gas-phase (red lines) and solid-state (blue lines) species (from Walsh et al (2014) © ESO).
) as a function of radius for gas-phase (red lines) and solid-state (blue lines) species (from Walsh et al (2014) © ESO).
Download figure:
Standard image High-resolution imageCOMs can, of course, be detected in interstellar clouds, most notably hot molecular cores, where the evidence is persuasive that there is a significant, if not dominant, contribution to their formation from chemistry occurring in the grain ices (section 1.1.4). The evidence includes the high degree of hydrogenation seen in these molecules, difficult to achieve through H-atom abstraction reactions involving  , their relatively high levels of deuterium fractionation, which indicates formation at low temperatures rather than in the high temperature gas in which they are detected, and very high fractional abundances, often many orders of magnitude above those seen in cold clouds.
, their relatively high levels of deuterium fractionation, which indicates formation at low temperatures rather than in the high temperature gas in which they are detected, and very high fractional abundances, often many orders of magnitude above those seen in cold clouds.
There have been a number of 'global' mechanisms proposed for such grain ice chemistry. These involve surface hydrogenation of simple, abundant gas-phase atoms and molecules following freeze-out on to the grains, and the interaction between the radicals that are intermediate species. Thus HCO, an intermediate in the hydrogenation of CO to form  OH (Watanabe et al 2004), is proposed to dimerise followed by H-atom addition to form glycolaldehyde,
OH (Watanabe et al 2004), is proposed to dimerise followed by H-atom addition to form glycolaldehyde,  OHCHO (Woods et al 2013). Woods et al have studied the dimerisation process via DFT calculations and found it to be barrierless and thus a potential source of glycolaldehyde. Dimerisation implies that the HCO radicals are sufficiently mobile to find one another on the surface and will, of course, compete with other reactions, including H addition to form
OHCHO (Woods et al 2013). Woods et al have studied the dimerisation process via DFT calculations and found it to be barrierless and thus a potential source of glycolaldehyde. Dimerisation implies that the HCO radicals are sufficiently mobile to find one another on the surface and will, of course, compete with other reactions, including H addition to form  CO and
CO and  OH as well as reaction with other mobile atoms and radicals. Garrod and Herbst (2006) have proposed that in warmer regions such as HMCs, the elevated temperatures, around 20–30 K, of the ice makes radicals sufficiently mobile, either thermally or via quantum tunnelling, that COMs form readily. While such processes may encourage the formation of COMs in warm or hot gas, the recent detection of COMs in cold sources, such as the low mass binary protostar IRAS 16 293–2422 (Jørgensen et al 2012), is more difficult to explain in this scenario. A further interesting aspect of this problem is that gylcolaldehyde has two other isomers detected in the interstellar medium, methy formate,
OH as well as reaction with other mobile atoms and radicals. Garrod and Herbst (2006) have proposed that in warmer regions such as HMCs, the elevated temperatures, around 20–30 K, of the ice makes radicals sufficiently mobile, either thermally or via quantum tunnelling, that COMs form readily. While such processes may encourage the formation of COMs in warm or hot gas, the recent detection of COMs in cold sources, such as the low mass binary protostar IRAS 16 293–2422 (Jørgensen et al 2012), is more difficult to explain in this scenario. A further interesting aspect of this problem is that gylcolaldehyde has two other isomers detected in the interstellar medium, methy formate,  , and acetic acid,
, and acetic acid,  COOH. Gas-phase and grain surface formation mechanisms have been suggested for all but it is as yet unclear which dominate, somthing that will require careful observational and experimental studies. Herbst and van Dishoeck (2009) have summarised the observations of and proposed synthetic pathways to COMs in a relatively recent review.
COOH. Gas-phase and grain surface formation mechanisms have been suggested for all but it is as yet unclear which dominate, somthing that will require careful observational and experimental studies. Herbst and van Dishoeck (2009) have summarised the observations of and proposed synthetic pathways to COMs in a relatively recent review.
As well as influencing local molecular abundances and therefore emission, the interaction between the gas and grain species can also have global impact. An important example, very relevant to the Solar System, is the sequestration of carbon in CO which can influence to a significant degree the amount of carbon available for organic molecules. Figure 12, taken from Lee et al (2010), shows the variation in the carbon/silicon ratio compared to the hydrogen/silicon ratio in a number of astronomical environments and shows that the amount of carbon in the Earth's mantle is highly depleted compared to the amount of carbon available at the formation of the Solar System, the Solar/ISM value. This appears to be true even if one takes into account that one is not able to measure the amount of carbon in the Earth's core. Indeed one sees that carbon depletion is also apparent in meteoritic bodies indicating that, more generally, it is difficult to incorporate carbon into refractory bodies despite the fact that the carbon abundance appears normal in the cooler materials that reside in the outer Solar System.
Figure 12. Carbon abundances, indicated by 'C', in the Solar System relative to silicon, showing that carbon is highly depleted in the Earth. Ratios are plotted for four different types of chondritic meteorite (from Lee et al (2010) © 2010. The American Astronomical Society.).
Download figure:
Standard image High-resolution imageLee et al (2010) suggest that carbon grains, transported into the inner Solar System, are eroded by thermally hot oxygen atoms in a region above the midplane, a region in which oxygen atoms are produced by photodissociation and grains are suspended by turbulent stirring. This chemical erosion occurs for carbon grains but not for silicate grains which survive to form the terrestrial planets. Since this process results in gas-phase carbon that either accretes on to the star or is lost to the system, the carbon found on Earth must be delivered, with water, in the form of carbon-bearing ices by cometary impacts.
An alternative explanation has been suggested by Favre et al (2013) who used Submillimetre Array (SMA) observations of CO and Herschel Space Observatory measurements of HD toward the TW Hya disk to show that gas-phase CO was depleted by a factor of around 10 compared to the expected C/H ratio in the warm molecular layer that is probed by the observations. Favre et al infer that CO is destroyed through reaction with  with the
with the  produced being processed into hydrocarbon molecules while the O atoms form
produced being processed into hydrocarbon molecules while the O atoms form  O. These molecules, which have larger binding energies to water ice than CO, freeze out on to grain surfaces and can be transported more efficiently to the inner PPD than the volatile CO molecule.
O. These molecules, which have larger binding energies to water ice than CO, freeze out on to grain surfaces and can be transported more efficiently to the inner PPD than the volatile CO molecule.
2.3. Deuterium fractionation
One of the surprising results of early radio astronomical observations of interstellar molecules was that D/H ratios in molecules such as  and HCN were enhanced significantly, or fractionated, over the cosmic D/H ratio, (D/H)
and HCN were enhanced significantly, or fractionated, over the cosmic D/H ratio, (D/H) , set by nucleosynthesis in the Big Bang and the limited amount of astration, i.e. the destruction of deuterium in stars, that has occurred since then. Table 4 lists those species detected to date in interstellar clouds.
, set by nucleosynthesis in the Big Bang and the limited amount of astration, i.e. the destruction of deuterium in stars, that has occurred since then. Table 4 lists those species detected to date in interstellar clouds.
Table 4. Deuterated molecules detected in interstellar clouds.
| Number of atoms | |||||||
|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| HD |  |
HDCO | c- HD HD |
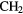 DOH DOH |
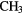 CCD CCD |
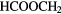 D D |
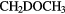 |
| ND | DCN | 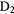 CO CO |
c-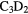 |
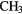 OD OD |
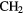 DCCH DCCH |
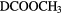 (?) (?) |
|
| OD | DNC | HDCS | 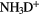 |
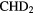 OH OH |
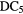 N N |
||
| HDO | 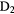 CS CS |
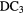 N N |
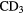 OH OH |
||||
| HDS | 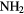 D D |
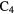 D D |
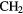 DCN DCN |
||||
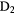 S S |
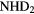 |
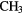 D(?) D(?) |
HDCCCC | ||||
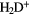 |
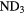 |
||||||
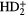 |
l-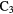 D D |
||||||
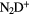 |
|||||||
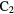 D D |
|||||||
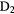 O O |
|||||||
It was quickly realised that quasi-stable molecular ions were key to understanding fractionation. Three major processes are initiated by D-fractionating reactions involving the 'bath' of deuterium in dense clouds, HD:
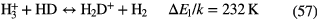
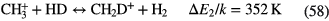
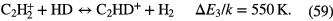
These reactions are exoergic in the forward direction by energies which are given for ground state reactants. Thus, at the low temperatures of cold dark clouds, the back reactions do not proceed and the  /
/ abundance ratio can be much larger than that of HD/
abundance ratio can be much larger than that of HD/ . To get an estimate of the enhancements possible, consider
. To get an estimate of the enhancements possible, consider  which has additional loss reactions through dissociative recombination with electrons and reaction with neutral species, M. Then, assuming steady state for simplicity
which has additional loss reactions through dissociative recombination with electrons and reaction with neutral species, M. Then, assuming steady state for simplicity
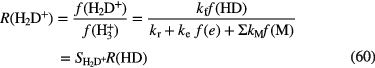
where  and
and  are the forward and reverse rate coefficients of (57),
are the forward and reverse rate coefficients of (57),  represents the fractional abundance,
represents the fractional abundance,  (
( ), of species X, M = CO, O,
), of species X, M = CO, O,  ,
,  O, etc and
O, etc and  (T) is the enhancement factor which is a function of T. For conditions typical of cold, dark clouds the third term in the denominator dominates and
(T) is the enhancement factor which is a function of T. For conditions typical of cold, dark clouds the third term in the denominator dominates and  , that is, R(
, that is, R( )
)  0.01–0.1. For
0.01–0.1. For  K, the back reaction with
K, the back reaction with  becomes important and R(
becomes important and R( ) decreases exponentially with increasing temperature—thus, this fractionation process is unimportant at room temperature.
) decreases exponentially with increasing temperature—thus, this fractionation process is unimportant at room temperature.
Radiative association of  with
with  and
and  competes with the back reactions in (58) and (59) so that the behaviours of R(
competes with the back reactions in (58) and (59) so that the behaviours of R( ) and R(
) and R( ) differ in detail from that of R(
) differ in detail from that of R( ). The general conclusion, however, is that each of the quasi-stable ions can be significantly fractionated in deuterium below a critical temperature that is approximately one-tenth of the value of the appropriate endothermicity.
). The general conclusion, however, is that each of the quasi-stable ions can be significantly fractionated in deuterium below a critical temperature that is approximately one-tenth of the value of the appropriate endothermicity.
This initial fractionation in the ions can be passed on to other species through subsequent reactions. Thus, in collision with CO,  can transfer either a deuteron or a proton, with statistically one-third of reactions producing
can transfer either a deuteron or a proton, with statistically one-third of reactions producing  rather than
rather than  :
:

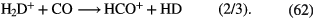
Since the loss mechanisms of  and
and  are essentially the same, this leads to the prediction that R(
are essentially the same, this leads to the prediction that R( )
)  R(
R( )/3 and similarly for other protonated ions such as
)/3 and similarly for other protonated ions such as  and
and  . The observational determination of the
. The observational determination of the  /
/ ratio in cold clouds thus leads to an estimate of the fractional ionization,
ratio in cold clouds thus leads to an estimate of the fractional ionization,  , an important parameter in controlling the star formation process. This can be achieved by using (60) since the rate coefficients and the CO abundance, the major contributor to species M, can be measured. In addition, and importantly, the dissociative recombination of
, an important parameter in controlling the star formation process. This can be achieved by using (60) since the rate coefficients and the CO abundance, the major contributor to species M, can be measured. In addition, and importantly, the dissociative recombination of  leads to an enhanced atomic D/H ratio. This enhancement can lead directly to a large fractionation in, for example, gas-phase OD:
leads to an enhanced atomic D/H ratio. This enhancement can lead directly to a large fractionation in, for example, gas-phase OD:
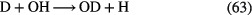
which is exothermic by about 800 K (Croswell and Dalgarno 1985). Croswell and Dalgarno predicted OD/OH ratios of a few percent in dark clouds. The large gas-phase atomic D/H ratio also leads, through collisions with grains, to an enhanced D/H ratio on ice mantles which can drive a D-rich chemistry on the surface. It is this chemistry that is believed to be the source of high D/H ratios measured in gas-phase molecules in hot molecular cores as discussed in section 1.1.4.
In very cold dense regions, all molecules, with the exception of hydrogen and its ions, will collide with and freeze-out on to dust grains on a rapid time-scale (section 1.3.2). This leads to the situation where the molecules that are routinely observed in order to derive physical conditions in dark clouds, for example, CO,  , HCN, CS,
, HCN, CS,  etc, are no longer present in the gas phase. In such regions, however, new species become abundant. In these objects, HD remains in the gas and can further deuterate
etc, are no longer present in the gas phase. In such regions, however, new species become abundant. In these objects, HD remains in the gas and can further deuterate  to
to  in the reaction chain:
in the reaction chain:
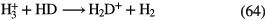
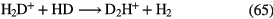
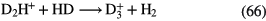
with the only competition being dissociative recombination with electrons. One finds that, under these conditions,  can be more abundant than
can be more abundant than  and that atomic D/H ratios in the gas and on the grains can be larger than unity. This has important implications for deuterating the trace amounts of neutral species that remain in the gas as well as driving fractionation via grain surface reactions. Thus, for example, Brown and Millar (1989) showed that the OD/OH ratio could be as large as unity due to the D + OH reaction. Recently this has been verified by the detection of OD at 1.392 THz using SOFIA, the Stratospheric Observatory for Infrared Astronomy, in the cold low-mass proto-binary source IRAS 16 293–2422, a source which contains very large abundances of multiply-deuterated molecules including
and that atomic D/H ratios in the gas and on the grains can be larger than unity. This has important implications for deuterating the trace amounts of neutral species that remain in the gas as well as driving fractionation via grain surface reactions. Thus, for example, Brown and Millar (1989) showed that the OD/OH ratio could be as large as unity due to the D + OH reaction. Recently this has been verified by the detection of OD at 1.392 THz using SOFIA, the Stratospheric Observatory for Infrared Astronomy, in the cold low-mass proto-binary source IRAS 16 293–2422, a source which contains very large abundances of multiply-deuterated molecules including  CO and
CO and  OH (Parise et al 2004, Parise et al 2012). Although it was not possible to derive the OD/OH abundance—OH is unobservable with SOFIA and although observable at centimetre wavelengths, these do not allow an accurate ratio to be derived since they sample completely different gas, both spatially and in density. Nevertheless, Parise et al infer a very large abundance of OD by comparing it to HDO with OD/HDO
OH (Parise et al 2004, Parise et al 2012). Although it was not possible to derive the OD/OH abundance—OH is unobservable with SOFIA and although observable at centimetre wavelengths, these do not allow an accurate ratio to be derived since they sample completely different gas, both spatially and in density. Nevertheless, Parise et al infer a very large abundance of OD by comparing it to HDO with OD/HDO  17–90. It is precisely in these cold (10 K), dense (
17–90. It is precisely in these cold (10 K), dense (
 ), regions that doubly and triply deuterated molecules, such as
), regions that doubly and triply deuterated molecules, such as  S,
S,  CO,
CO,  OH, and
OH, and  , are observed. Figure 13, from Roberts and Millar (2006), shows the fractional abundances and D/H abundance ratios for a number of species for the particular case of a cold, dense cloud in which accretion onto grains occurs on a time-scale of about
, are observed. Figure 13, from Roberts and Millar (2006), shows the fractional abundances and D/H abundance ratios for a number of species for the particular case of a cold, dense cloud in which accretion onto grains occurs on a time-scale of about  yrs. It shows that D/H ratios in the hydride ions can reach extremely large values, greater than unity, and can enhance the fractionation in other molecules; the abundance ratio of
yrs. It shows that D/H ratios in the hydride ions can reach extremely large values, greater than unity, and can enhance the fractionation in other molecules; the abundance ratio of  /
/ , for example, is greater than 0.1 at cloud ages beyond 40 000 yrs.
, for example, is greater than 0.1 at cloud ages beyond 40 000 yrs.
Figure 13. Fractional abundances (a) and D/H ratios (b) calculated for a variety of molecules for a model in which  K and n(
K and n( ) =
) = 
 (from Roberts and Millar (2006)).
(from Roberts and Millar (2006)).
Download figure:
Standard image High-resolution imageThese regions of enhanced accretion on to grains do allow for the presence of newly observable species to probe the physics of the gas, namely  and
and  , both of which possess small permanent electric dipole moments, and have allowed rotational transitions in the sub-millimetre range, unlike
, both of which possess small permanent electric dipole moments, and have allowed rotational transitions in the sub-millimetre range, unlike  and
and  . The new generation of sub-millimetre telescopes on high dry sites, such as the single-dish APEX telescope and the ALMA 66-dish interferometer situated at an altitude of 5000 m in the Atacama Desert, (figure 14), will finally enable us to study the end point of the collapse of molecular gas to form stars.
. The new generation of sub-millimetre telescopes on high dry sites, such as the single-dish APEX telescope and the ALMA 66-dish interferometer situated at an altitude of 5000 m in the Atacama Desert, (figure 14), will finally enable us to study the end point of the collapse of molecular gas to form stars.
Figure 14. A night-time view of the 66-element ALMA interferometer. The two irregular galaxies in the picture are the Large and Small Magellanic Clouds. (Photo credit: ESO.org Christoph Malin).
Download figure:
Standard image High-resolution imageThe detailed fractionation driven by  and
and  was first described by Roberts et al (2003) but it was Flower et al (2004) who noted that the detailed energetics of the reactions leading to
was first described by Roberts et al (2003) but it was Flower et al (2004) who noted that the detailed energetics of the reactions leading to  would be affected by the rotational states, ortho, para and meta, occupied by the reactants and by nuclear spin conservation rules (Oka 2004, Hugo et al 2009). As an example, at 10 K,
would be affected by the rotational states, ortho, para and meta, occupied by the reactants and by nuclear spin conservation rules (Oka 2004, Hugo et al 2009). As an example, at 10 K,  can be found either in its
can be found either in its  (ortho) or
(ortho) or  (para) states with
(para) states with  K—radiative transitions between
K—radiative transitions between  and
and  states are not allowed. Thus the back reactions of (57)–(59) possess energy barriers that are significantly reduced for collisions involving
states are not allowed. Thus the back reactions of (57)–(59) possess energy barriers that are significantly reduced for collisions involving  . This would not be significant in cold (10 K) clouds if
. This would not be significant in cold (10 K) clouds if  was in local thermodynamic equilibrium but this does not appear likely (the rotational populations of
was in local thermodynamic equilibrium but this does not appear likely (the rotational populations of  cannot be determined directly in these regions since
cannot be determined directly in these regions since  is unobservable). The excess energy, 4.5 eV, associated with
is unobservable). The excess energy, 4.5 eV, associated with  formation on dust grains sets the initial ortho-para ratio in
formation on dust grains sets the initial ortho-para ratio in  to 3:1, the high temperature limit. The ortho-para ratio can be altered through proton exchange reactions of
to 3:1, the high temperature limit. The ortho-para ratio can be altered through proton exchange reactions of  and
and  with
with  but this is a slow process at the low temperatures and low ionization levels in dark clouds, and takes much longer than
but this is a slow process at the low temperatures and low ionization levels in dark clouds, and takes much longer than  yr, i.e. significantly greater than the cloud lifetime,
yr, i.e. significantly greater than the cloud lifetime,  yr, to reach the LTE value of
yr, to reach the LTE value of  (Pagani et al 2009). In dark clouds the ortho-para ratio of
(Pagani et al 2009). In dark clouds the ortho-para ratio of  is likely between 0.1 and 0.001, sufficient to decrease the fractionation of
is likely between 0.1 and 0.001, sufficient to decrease the fractionation of  and related ions from the very high values given by the simple approximations above.
and related ions from the very high values given by the simple approximations above.
Although inclusion of quantum effects lowers the efficiency of fractionation in the gas phase, the fractionation of  ,
,  and
and  leads to large atomic D/H ratios and thus to large fractionation in OD via the gas-phase reaction (63) and on grain ice mantles following accretion of D atoms. The influence of nuclear spin on the fractionation of the hydrogen species is shown in figure 15 (Sipilä et al 2010) which compares the steady-state abundance ratios as a function of density for two sets of state-to-state rate coefficients (Flower et al 2004, Hugo et al 2009). These results are equivalent to the long-time evolution in the calculations by Roberts and Millar (2006), that is, in the regime where all elements heavier than H and He are removed from the gas.
leads to large atomic D/H ratios and thus to large fractionation in OD via the gas-phase reaction (63) and on grain ice mantles following accretion of D atoms. The influence of nuclear spin on the fractionation of the hydrogen species is shown in figure 15 (Sipilä et al 2010) which compares the steady-state abundance ratios as a function of density for two sets of state-to-state rate coefficients (Flower et al 2004, Hugo et al 2009). These results are equivalent to the long-time evolution in the calculations by Roberts and Millar (2006), that is, in the regime where all elements heavier than H and He are removed from the gas.
Figure 15. Steady-state fractional abundances calculated at 10K as a function of density for two choices of the state-to-state rate coefficients (from Sipilä et al (2010) © ESO).
Download figure:
Standard image High-resolution imageThese plots confirm the results of Roberts and Millar, namely that very large fractionation ratios can arise in dense, cold gas when heavy elements are frozen out on to grain surfaces. They show that, in addition, the detailed abundances depend on the specific rotational populations and the spin-selection rules applied to the reactions. As shown by Sipilä et al, an accurate calculation of the state-to-state chemistry is essential to accurately interpret observations of  and
and  in dense cores. If the grains play only a passive role in influencing gas-phase molecular abundances, that is simply through acting as a sink for molecules, then it is is clear that these deuterated ions give us a unique probe of the late stages of cloud collapse and the early stages of star formation. The situation is less clear, however, if deuterium takes part in an active surface chemistry. Sipilä et al (2013) have argued that D atoms, which will be heavily fractionated relative to H atoms due to recombination of abundant
in dense cores. If the grains play only a passive role in influencing gas-phase molecular abundances, that is simply through acting as a sink for molecules, then it is is clear that these deuterated ions give us a unique probe of the late stages of cloud collapse and the early stages of star formation. The situation is less clear, however, if deuterium takes part in an active surface chemistry. Sipilä et al (2013) have argued that D atoms, which will be heavily fractionated relative to H atoms due to recombination of abundant  for example, will react on icy surfaces to form HDO and
for example, will react on icy surfaces to form HDO and  O rather than re-cycle to HD. The net effect is to reduce the abundance of HD in the gas, since the deuterated ions are produced in reactions that involve HD. The outcome can be severe—Sipilä et al show that the HD abundance can be reduced by two to three orders of magnitude between
O rather than re-cycle to HD. The net effect is to reduce the abundance of HD in the gas, since the deuterated ions are produced in reactions that involve HD. The outcome can be severe—Sipilä et al show that the HD abundance can be reduced by two to three orders of magnitude between  and
and  yrs for gas at a density of n(
yrs for gas at a density of n(
 . This has very significant implications for the chemistry since all deuterated species will be depleted in the high density cores of clouds—Sipilä et al find that fractionation is larger at core edges than at core centres—and that HD can not be used as a proxy for the
. This has very significant implications for the chemistry since all deuterated species will be depleted in the high density cores of clouds—Sipilä et al find that fractionation is larger at core edges than at core centres—and that HD can not be used as a proxy for the  abundance and mass, an assumption used by Favre et al (2013) in their study of CO depletion in the TW Hya PPD as discussed in section 1.1.3. If HD is, in fact, depleted in the disk region probed by the SMA, then the CO/
abundance and mass, an assumption used by Favre et al (2013) in their study of CO depletion in the TW Hya PPD as discussed in section 1.1.3. If HD is, in fact, depleted in the disk region probed by the SMA, then the CO/ ratio in the gas may be normal and the extraction of carbon from CO and its incorporation into hydrocarbons may not be the explanation the carbon abundance anomaly in the Solar System. The detailed surface chemistry of deuterium is thus an important topic for laboratory investigation.
ratio in the gas may be normal and the extraction of carbon from CO and its incorporation into hydrocarbons may not be the explanation the carbon abundance anomaly in the Solar System. The detailed surface chemistry of deuterium is thus an important topic for laboratory investigation.
2.4. Isotopic fractionation
Enhancements of heavy isotopes of other elements are observed in interstellar molecules but are generally much less than those of D because of smaller zero-point energy differences. In diffuse clouds, for example, where photoionization ensures that  is the dominant isotope bath, the reaction:
is the dominant isotope bath, the reaction:
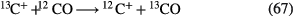
with  K, enables
K, enables  CO/
CO/ CO to be larger than the
CO to be larger than the  C/
C/ C elemental ratio. The effect is not large however and is limited in such clouds because photodissociation of CO occurs though the line absorption of UV photons, with the result that
C elemental ratio. The effect is not large however and is limited in such clouds because photodissociation of CO occurs though the line absorption of UV photons, with the result that  CO self-shields, i.e. the particular UV photons capable of dissociating
CO self-shields, i.e. the particular UV photons capable of dissociating  CO are absorbed, before
CO are absorbed, before  CO, which has a smaller abundance, self-shields. Thus, the photodissociation rate of
CO, which has a smaller abundance, self-shields. Thus, the photodissociation rate of  CO is slightly larger than that of
CO is slightly larger than that of  CO, thereby reducing the
CO, thereby reducing the  CO enhancement driven by the
CO enhancement driven by the  reaction. The overall outcome is that
reaction. The overall outcome is that  CO/
CO/ CO is slightly enhanced, and that of
CO is slightly enhanced, and that of  /
/ slightly decreased, below the cosmic (or local) value. As is the case for deuterium, this enhancement/depletion can be passed on to other species depending on the chemical network. Thus
slightly decreased, below the cosmic (or local) value. As is the case for deuterium, this enhancement/depletion can be passed on to other species depending on the chemical network. Thus  will reflect the CO isotopic ratio since it is formed directly by the proton transfer reaction:
will reflect the CO isotopic ratio since it is formed directly by the proton transfer reaction:
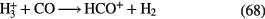
whilst other species, for example CN and HCN, formed via reactions involving simple hydrocarbons such as CH and  , reflect the depleted ratio in
, reflect the depleted ratio in  .
.
Fractionation can also occur in O, N, and S although the effects are small and difficult to measure unambiguously through molecular line observations.
2.5. Grain surface chemistry
As indicated in section 1.3.2, it is expected that all species, with the exception of  , He and their ions, will freeze out on to the surface of cold dust grains on relatively short timescales in dark clouds. For a grain of radius 0.1
, He and their ions, will freeze out on to the surface of cold dust grains on relatively short timescales in dark clouds. For a grain of radius 0.1  m and around
m and around  binding sites on its surface, the removal of all O, C and N species from the gas will result in the growth of around 200 layers of 'ice'. This ice mantle is most easily observed through infrared absorption spectroscopy which traces vibrational transitions. Since one needs a bright infrared star behind or embedded within a cold, dark cloud to perform the observations, and because the Earth's atmosphere absorbs IR photons, relatively few objects have been well-studied using ground-based telescopes. The advent of orbiting observatories, such as the Infrared Space Observatory, ISO, and in particular the Spitzer Space Telescope, have enabled detailed studies of ice composition in a variety of objects. As mentioned previously, one important limitation is that detections are sensitive only to fractional abundances that are greater than
binding sites on its surface, the removal of all O, C and N species from the gas will result in the growth of around 200 layers of 'ice'. This ice mantle is most easily observed through infrared absorption spectroscopy which traces vibrational transitions. Since one needs a bright infrared star behind or embedded within a cold, dark cloud to perform the observations, and because the Earth's atmosphere absorbs IR photons, relatively few objects have been well-studied using ground-based telescopes. The advent of orbiting observatories, such as the Infrared Space Observatory, ISO, and in particular the Spitzer Space Telescope, have enabled detailed studies of ice composition in a variety of objects. As mentioned previously, one important limitation is that detections are sensitive only to fractional abundances that are greater than  relative to
relative to  or about 0.1–1% of the abundance of water ice.
or about 0.1–1% of the abundance of water ice.
A typical IR spectrum of a cold dark cloud is shown in figure 4 with the main absorption bands identified. Table 5 presents the derived abundances toward three types of sources, low- and high-mass protostars embedded in molecular clouds and toward background sources, i.e. stars which lie beyond the molecular cloud and which cannot be influencing the composition and characteristics of ice mantles (Öberg et al 2011). Water ice is found to be dominant with CO,  and
and  OH the next most abundant species. Abundances of these and other more minor species such as
OH the next most abundant species. Abundances of these and other more minor species such as  CO, HCOOH,
CO, HCOOH,  and
and  , vary from object to object, perhaps reflecting local conditions and time scales. Table 5 lists the relative abundances of the most common species in ices. Detailed comparison of the interstellar ice band profiles with laboratory experimental data leads to the conclusion that interstellar ices are segregated or layered and that CO, for example, is found in both non-polar (dominated by CO and
, vary from object to object, perhaps reflecting local conditions and time scales. Table 5 lists the relative abundances of the most common species in ices. Detailed comparison of the interstellar ice band profiles with laboratory experimental data leads to the conclusion that interstellar ices are segregated or layered and that CO, for example, is found in both non-polar (dominated by CO and  ) and polar (predominantly
) and polar (predominantly  O) components.
O) components.
Table 5. Abundance medians for components of individual ices and individual ice components toward low- and high-mass protostars and through dark clouds toward background stars (from Öberg et al (2011)).
| Ice | Low mass | High mass | Background |
|---|---|---|---|
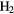 O O |
100 | 100 | 100 |
| CO | 38 | 13 | 31 |
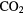 |
29 | 13 | 38 |
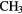 OH OH |
7 | 8 | 8 |
 |
5 | 15 | — |
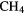 |
5 | 4 | — |
| XCN | 0.6 | 0.8 | — |
| Pure CO | 21 | 3 | — |
CO: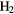 O O |
13 | 10 | — |
CO: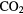 |
2 | 1.3 | — |
Pure 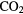 |
2 | 2 | — |
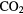 : :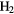 O O |
20 | 9 | 24 |
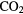 :CO :CO |
5 | 5 | 6 |
The most important reactive species on cold grain surfaces is atomic hydrogen due to its low binding energy and its ability to diffuse, either thermally or quantum mechanically, across the grain. To a first approximation, atomic hydrogen can be considered mobile, with heavier species fixed to particular surface sites. This, together with its relatively high abundance, means that, for example, O atoms accreting on to the surface are rapidly hydrogenated to form water, consistent with the high fractional abundance seen in the IR absorption bands—around 100 times more water is contained in ice than in the gas phase.
An accurate description of a detailed grain surface chemistry is made more difficult because of the number of fundamental issues which are very poorly understood at present. These include the detailed physical properties of the underlying grain, its composition, morphology, porosity, distribution of surface sites and their binding energies, surface versus bulk chemistry, and by processes which at first glance may be too slow to be significant, but which may not be so given typical ice mantle lifetimes of  yr in cold clouds. These processes include the interaction of cosmic rays and UV radiation with the dust.
yr in cold clouds. These processes include the interaction of cosmic rays and UV radiation with the dust.
Once the nature of the grain and its surface have been defined (at least for modelling purposes), one can begin to describe the chemical processes that occur which are typically one of two mechanisms: (i) Eley–Rideal, in which an incoming gas-phase reactant collides directly with an absorbed reactant, and (ii) Langmuir–Hinshelwood, in which two reactants interact following diffusion, either thermally or quantum mechanically. Mechanism (ii) therefore requires at least one of the reactants to be bound to the surface through physisorption rather than chemisorption. Under normal cold dark cloud conditions, that is, where grains are covered in ices, physisorption and diffusion dominates and therefore reactions involving mobile H and D atoms tend to dominate the chemistry.
A final fundamental issue is that rate equations, routinely used to describe gas-phase chemical kinetics, may not be appropriate when describing surface chemistry on interstellar grains, since the use of average concentrations, central to the rate equation description, has limited use in kinetics when the average number of reactive species becomes less than one per grain. Thus, for example, a grain containing one H atom on its surface cannot form  , whereas an approach using the average surface concentration, the rate equation approach, would calculate a non-zero rate coefficient for
, whereas an approach using the average surface concentration, the rate equation approach, would calculate a non-zero rate coefficient for  formation. In addition, if the diffusion rate of a particle on the surface is large and the gas density small, so that collisions with the grain are rare, the surface particle is likely to react with the first particle that lands on the grain, the so-called 'accretion limit'.
formation. In addition, if the diffusion rate of a particle on the surface is large and the gas density small, so that collisions with the grain are rare, the surface particle is likely to react with the first particle that lands on the grain, the so-called 'accretion limit'.
The most accurate way to treat surface chemistry in this limit is to use stochastic methods, that is, through solving the master equation, in which one describes the change in time of the probability density function of this system. Here, one associates a probability density  with state
with state  at time t, where
at time t, where  =
= ![$\left[{{N}_{1}}(t),{{N}_{2}}(t),....,{{N}_{k}}(t)\right]$](https://content.cld.iop.org/journals/0963-0252/24/4/043001/revision1/psst514885ieqn749.gif) is described by the number of particles,
is described by the number of particles,  (t), of each species at time t, and which changes with time due to chemical reactions. Thus, for example, chemistry on a surface can be represented by chemistry on a set of lattice points, each corresponding to a surface site, with the population at each point described by the state vector
(t), of each species at time t, and which changes with time due to chemical reactions. Thus, for example, chemistry on a surface can be represented by chemistry on a set of lattice points, each corresponding to a surface site, with the population at each point described by the state vector  .
.
Because of the stochastic nature of this approach, computational time can be long and several approximations are normally made in interstellar studies:—(i) all sites are identical, so one can follow the total number of each species on the surface, and (ii) the distribution of particles on the surface is random. The terms equivalent to rate coefficients in gas-phase chemistry then depend, for reactive species i and j, on the times taken for the species to scan the grain surface, on the presence of an activation energy barrier, and on a combinatorial factor.
One sees that a stochastic approach can lead to an 'infinite' number of possible states, if, for example, one associates a probability to every possible state of the system. One can reduce the equations to a finite set by imposing a 'maximum population', a valid approximation given that the stochastic approach is needed only when the number of reactants is small. Solution of the master equation then gives the probabilities for each of these finite number of states from which the expectation values  of each species can be derived. Such an approach is computationally intensive, particularly when compared to a gas-phase chemistry, and is limited to the treatment of only a few surface species (e.g. Stantcheva and Herbst (2004)).
of each species can be derived. Such an approach is computationally intensive, particularly when compared to a gas-phase chemistry, and is limited to the treatment of only a few surface species (e.g. Stantcheva and Herbst (2004)).
An alternative to the direct solution of the master equation is to use Monte Carlo methods although a large number of model calculations must be made in order to obtain convergence (Caselli et al 1998, Charnley 1998). Cuppen and Herbst (2005) and (2007) have pioneered the use of Continuous Time Random Walk (CTRW) microscopic Monto Carlo methods in astrochemistry. In their work, they follow the path of every particle on every site in a model surface. Their description allowed for site-specific properties, e.g. a variety of binding energies, and for multi-layer growth of ice on the surface, and considered both  formation and the growth of ice mantles. Cuppen and Garrod (2011) used the same technique to investigate
formation and the growth of ice mantles. Cuppen and Garrod (2011) used the same technique to investigate  formation on dust grains containing surface irregularities that is, sites possessing different binding energies, and have shown that the modified rate equations can be used to reproduce the CTRW results as long as the population of 'strong' binding sites is less than about 30%.
formation on dust grains containing surface irregularities that is, sites possessing different binding energies, and have shown that the modified rate equations can be used to reproduce the CTRW results as long as the population of 'strong' binding sites is less than about 30%.
The major drawback of such microscopic models is their computational expense which limits the time scales and number of species that can be described. Vasyunin and Herbst (2013) have described a macroscopic Monte Carlo model that incorporates both gas phase and grain surface reactions, in total around 6000 reactions describing the chemistry of 660 species. They argue that their results, which are calculated for times up to  yr, are more exact although computational times are still long. One approach to reducing computational effort was introduced by Lipshtat and Biham (2004) who noted that the chemical system is sparse, that is, most species react with only a few others. If it is assumed that unreactive species are conditionally independent of each other, than the number of equations in the master equation approach can be reduced dramatically. This multiplane method has been shown by Barzel et al (2010) to be in good agreement with the direct solution of the master equation. Barzel and Biham (2011) also showed that moment equations generated from the master equation could be used in an efficient manner. The generation of moment equations results in the problem that higher-order moments appear, thus making closure difficult. Barzel and Biham (2011) and Barzel and Biham (2012) showed that the moment equations could be expressed in terms of so-called binomial moments, linear combinations of ordinary moments, which captured the essential physics, but allowed for a much-reduced set of linear equations, rising polynomially with the number of reactive species, rather than the exponential increase which results when using the master equation approach. The moment method generates one equation for each species and one equation for each reaction. While ordinary moment equations are often adequate, Barzel and Biham (2012) show that binomial moments allow for a greater range of chemical processes, have an intuitive physical meaning and lead to easily writable forms of the equations, while giving excellent agreement with the more complete Monte Carlo methods.
yr, are more exact although computational times are still long. One approach to reducing computational effort was introduced by Lipshtat and Biham (2004) who noted that the chemical system is sparse, that is, most species react with only a few others. If it is assumed that unreactive species are conditionally independent of each other, than the number of equations in the master equation approach can be reduced dramatically. This multiplane method has been shown by Barzel et al (2010) to be in good agreement with the direct solution of the master equation. Barzel and Biham (2011) also showed that moment equations generated from the master equation could be used in an efficient manner. The generation of moment equations results in the problem that higher-order moments appear, thus making closure difficult. Barzel and Biham (2011) and Barzel and Biham (2012) showed that the moment equations could be expressed in terms of so-called binomial moments, linear combinations of ordinary moments, which captured the essential physics, but allowed for a much-reduced set of linear equations, rising polynomially with the number of reactive species, rather than the exponential increase which results when using the master equation approach. The moment method generates one equation for each species and one equation for each reaction. While ordinary moment equations are often adequate, Barzel and Biham (2012) show that binomial moments allow for a greater range of chemical processes, have an intuitive physical meaning and lead to easily writable forms of the equations, while giving excellent agreement with the more complete Monte Carlo methods.
While there are a variety of stochastic approaches developed for situations such as cellular chemistry, where the number of reactant molecules can also be small or not well-mixed—see Gillespie et al (2013) for a review of these techniques—their use in astrochemical models is still at an exploratory level. The interested reader is referred to a recent review by Cuppen et al (2013) on the use of stochastic models in describing surface chemistry on interstellar dust grains.
One of the key motivations behind such studies is to discover whether the fundamental chemistry that drove the emergence of life on Earth was restricted to the planet itself or whether it could have had a contribution from chemical processes that occurred in space, either in molecular clouds or in protoplanetary disks. Interstellar and cometary ices are a potential delivery mechanism so a number of experiments have been undertaken, involving thermal and non-thermal processes, to answer this question. Indeed gylcine, confirmed as extraterrestrial in origin through its  C/
C/ C ratio, has been detected in the material sampled by the Stardust comet return mission to comet 81P/Wild 2 (Elsila et al 2009). There are a range of non-thermal processes which are known to drive molecular synthesis. Thus, the interaction of UV and x-ray photons and high-energy particles such as electrons and ions with ices leads to the formation of quite complex species in the ice. Ciaravella et al (2012) have irradiated pure CO ices with soft x-rays and find the formation of a range of carbon oxides including
C ratio, has been detected in the material sampled by the Stardust comet return mission to comet 81P/Wild 2 (Elsila et al 2009). There are a range of non-thermal processes which are known to drive molecular synthesis. Thus, the interaction of UV and x-ray photons and high-energy particles such as electrons and ions with ices leads to the formation of quite complex species in the ice. Ciaravella et al (2012) have irradiated pure CO ices with soft x-rays and find the formation of a range of carbon oxides including  ,
,  O,
O,  and
and  O. An interesting set of complex organic molecules is formed if methanol ice is used. Chen et al (2013) detect the presence of
O. An interesting set of complex organic molecules is formed if methanol ice is used. Chen et al (2013) detect the presence of  OH,
OH,  , acetic acid
, acetic acid  COOH, methyl formate
COOH, methyl formate  , dimethyl ether
, dimethyl ether  and ethylene glycol (
and ethylene glycol ( O)2, all observed interstellar molecules, amongst others. Here the chemistry is mediated by the generation of secondary electrons with an energy distribution that is dependent on the energy of the x-ray photons. The interaction of x-rays with ice mantles is likely to be important in PPDs (section 1.1.3) since young stars can be strong emitters of x-rays and the penetration depth into the disk is larger for x-rays than for UV photons, which can also generate complex species when they interact with methanol ice (Öberg et al 2009). Ongoing experiments are needed, not only to investigate the formation of complex molecules but also to see if the kinetics of their formation can be understood through either the use of deterministic or stochastic models. If successful, this would provide a degree of comfort in extending models to the interstellar environment where interactions with the grains occur at the single photon/particle level.
O)2, all observed interstellar molecules, amongst others. Here the chemistry is mediated by the generation of secondary electrons with an energy distribution that is dependent on the energy of the x-ray photons. The interaction of x-rays with ice mantles is likely to be important in PPDs (section 1.1.3) since young stars can be strong emitters of x-rays and the penetration depth into the disk is larger for x-rays than for UV photons, which can also generate complex species when they interact with methanol ice (Öberg et al 2009). Ongoing experiments are needed, not only to investigate the formation of complex molecules but also to see if the kinetics of their formation can be understood through either the use of deterministic or stochastic models. If successful, this would provide a degree of comfort in extending models to the interstellar environment where interactions with the grains occur at the single photon/particle level.
2.6. Shock waves in molecular clouds
It has been known for around 40 years that molecular emission lines, particularly those in regions of star formation, have line widths that can be highly supersonic, up to several hundred km  in some cases. The presence of such motions, which can often be traced through ro-vibrational emission from
in some cases. The presence of such motions, which can often be traced through ro-vibrational emission from  , one of the very few instances in which
, one of the very few instances in which  is visible to our telescopes, is likely due to the propagation of pressure disturbances or shock waves through interstellar gas. These can be generated by a multiplicity of processes, including stellar jets and winds, cloud-cloud collisions, and stellar explosions, such as novae and supernovae. Since shocks compress and heat the gas, they can induce chemical reactions that would not otherwise occur and cause both excitation and abundance conditions such that we can probe the shock physics in some detail. Shocks can also disrupt, and indeed destroy, interstellar grains through both sputtering and grain-grain collisions. Such destruction can return large amounts of heavy elements such as silicon to the gas. As this is readily converted to SiO, emission from this molecule acts as a good tracer of grain erosion in shock waves.
is visible to our telescopes, is likely due to the propagation of pressure disturbances or shock waves through interstellar gas. These can be generated by a multiplicity of processes, including stellar jets and winds, cloud-cloud collisions, and stellar explosions, such as novae and supernovae. Since shocks compress and heat the gas, they can induce chemical reactions that would not otherwise occur and cause both excitation and abundance conditions such that we can probe the shock physics in some detail. Shocks can also disrupt, and indeed destroy, interstellar grains through both sputtering and grain-grain collisions. Such destruction can return large amounts of heavy elements such as silicon to the gas. As this is readily converted to SiO, emission from this molecule acts as a good tracer of grain erosion in shock waves.
Consider, for the moment, a plane-parallel, hydrodynamic shock wave propagating at velocity  through gas in which the magnetic field,
through gas in which the magnetic field,  , is neglected. In the frame of reference in which the shock front is stationary, the relationship between the upstream and downstream properties depend only on the distance from the shock front and can be found by solving the Rankine–Hugoniot conditions for the conservation of mass, momentum and energy. For a pressure-density relation of the form
, is neglected. In the frame of reference in which the shock front is stationary, the relationship between the upstream and downstream properties depend only on the distance from the shock front and can be found by solving the Rankine–Hugoniot conditions for the conservation of mass, momentum and energy. For a pressure-density relation of the form  and a strong shock, defined by the Mach number
and a strong shock, defined by the Mach number  , where
, where  is the sound speed, one finds
is the sound speed, one finds  , and
, and
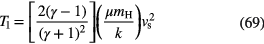
where subscript '0' refers to upstream gas and '1' to downstream gas. Thus for an adiabatic shock in atomic or cold molecular hydrogen gas,  and the gas density increases by a factor of 4 as the gas traverses the shock front. Its immediate post-shock velocity is 0.75
and the gas density increases by a factor of 4 as the gas traverses the shock front. Its immediate post-shock velocity is 0.75 and post-shock temperature
and post-shock temperature  K for atomic gas and
K for atomic gas and  3400 K for
3400 K for  for
for  km
km  . For an isothermal shock, in which the excess energy is radiated away, then the post-shock density is proportional to the Mach number of the shock and can be very large. Such highly compressed gas moves at the shock speed and forms a thin shell, with a typical width of
. For an isothermal shock, in which the excess energy is radiated away, then the post-shock density is proportional to the Mach number of the shock and can be very large. Such highly compressed gas moves at the shock speed and forms a thin shell, with a typical width of  cm, of swept-up, high density gas at the shock front.
cm, of swept-up, high density gas at the shock front.
For a shock propagating into  gas in which rotations but not vibrations are excited, that is, for
gas in which rotations but not vibrations are excited, that is, for  –6000 K,
–6000 K,  , the gas density increases by a factor of 6 and
, the gas density increases by a factor of 6 and  K for a 10 km
K for a 10 km  shock.
shock.
Magnetic fields generally play a role in the propagation of shocks since the interstellar gas is always ionized to some degree by UV photons, x-rays and cosmic ray particles. When the ionization fraction is high and the charged particles are well-coupled to each other, to the neutral gas and to the magnetic field, the 'frozen field' approximation is often adopted. This depends on the ions, electrons and neutral particles being well-coupled through collisions that transfer momentum between the gas particles. While ion-electron collisions in dense interstellar clouds are fast, the low fractional ionization,  , for a cloud with number density,
, for a cloud with number density, 
 , means that the coupling between ions and neutrals is weak and the compression of the magnetic field by the shock is not transmitted to the neutral gas. The poor coupling between ions and neutrals results in an extended region, much larger than the neutral-neutral collision length, in which ion magnetosonic waves propagate at velocities,
, means that the coupling between ions and neutrals is weak and the compression of the magnetic field by the shock is not transmitted to the neutral gas. The poor coupling between ions and neutrals results in an extended region, much larger than the neutral-neutral collision length, in which ion magnetosonic waves propagate at velocities,  , larger than the shock velocity
, larger than the shock velocity  , the so-called 'magnetic precursor', in which the charged and neutral fluids have different temperatures and velocities. In particular, for conditions appropriate to the interstellar medium, the shock does not cause a discontinuity—a jump or J-shock—and physical parameters in the pre- and post-shock gas are connected in a continuous manner, a so-called C-shock. The presence of a C-shock can have important consequences for the chemistry since ion-neutral streaming can overcome activation energy barriers and reaction endothermicities and the interaction region, L, can be much larger than that in hydrodynamic J-shocks. The length scale is given by Flower et al (1986) and Draine (1980):
, the so-called 'magnetic precursor', in which the charged and neutral fluids have different temperatures and velocities. In particular, for conditions appropriate to the interstellar medium, the shock does not cause a discontinuity—a jump or J-shock—and physical parameters in the pre- and post-shock gas are connected in a continuous manner, a so-called C-shock. The presence of a C-shock can have important consequences for the chemistry since ion-neutral streaming can overcome activation energy barriers and reaction endothermicities and the interaction region, L, can be much larger than that in hydrodynamic J-shocks. The length scale is given by Flower et al (1986) and Draine (1980):
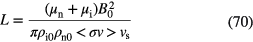
where  are the neutral and ionized masses respectively. Since
are the neutral and ionized masses respectively. Since  , L can be quite large for the low-ionized environments of molecular clouds.
, L can be quite large for the low-ionized environments of molecular clouds.
Indeed, there is important feedback from the chemistry on the shock structure since fast ion-neutral chemistry can lead to additional neutralisation of the gas. An example is the endothermic reaction:
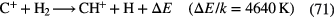
followed by:
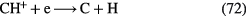
which leads, in diffuse molecular gas in which  is the most abundant ion, to a reduction in the overall fractional ionization of the gas.
is the most abundant ion, to a reduction in the overall fractional ionization of the gas.
Molecular gas cools very rapidly once the shock front passes, on a time scale of a few tens of years, so any region of hot, very dense, shock-processed gas is relatively thin by astronomical standards. For example, for a non-MHD shock, one can calculate analytically the column density of  , N(
, N( ), in the post-shock gas, that is, the number density of
), in the post-shock gas, that is, the number density of  per unit volume integrated along the line of sight through the shock front, if one assumes that it is formed by reaction (71) above and destroyed by exothermic reaction with
per unit volume integrated along the line of sight through the shock front, if one assumes that it is formed by reaction (71) above and destroyed by exothermic reaction with  (which is faster than dissociative recombination with electrons in many cases) then one finds:
(which is faster than dissociative recombination with electrons in many cases) then one finds:
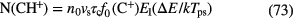
where  is the pre-shock number density (
is the pre-shock number density ( ),
),  is the shock velocity,
is the shock velocity,  the cooling time,
the cooling time,  (
( ), the initial, pre-shock fractional abundance of
), the initial, pre-shock fractional abundance of  ,
,  (y) the exponential integral of the first kind, and
(y) the exponential integral of the first kind, and  the immediate post-shock temperature. Here we have assumed that the post-shock gas cools exponentially from
the immediate post-shock temperature. Here we have assumed that the post-shock gas cools exponentially from  in time
in time  . Since
. Since  (
( ), the column density of
), the column density of  swept up by the shock front in one cooling time, is given by:
swept up by the shock front in one cooling time, is given by:
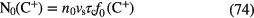
we see that the value of the exponential integral measures the efficiency by which  is converted to
is converted to  .
.
Pineau des Forêts et al (1986) have calculated the column density of  produced in transverse hydrodynamic and MHD shocks impinging on diffuse interstellar clouds, that is, gas at low number density such that UV photons are able to maintain a rather high fractional ionization,
produced in transverse hydrodynamic and MHD shocks impinging on diffuse interstellar clouds, that is, gas at low number density such that UV photons are able to maintain a rather high fractional ionization,  , in the pre-shock gas. For a hydrodynamic shock speed of 10 km
, in the pre-shock gas. For a hydrodynamic shock speed of 10 km  and an initial density of 20 hydrogen nuclei
and an initial density of 20 hydrogen nuclei  , they calculate an immediate post-shock temperature of 3060 K and a column density, N(
, they calculate an immediate post-shock temperature of 3060 K and a column density, N(
 for physical conditions appropriate for clouds in which the column density is observed to be greater than
for physical conditions appropriate for clouds in which the column density is observed to be greater than 
 .
.
Pineau des Forêts et al (1986) also calculated the effect of including a magnetic induction of  –12
–12  G. The neutrals, ions and electrons can thus possess independent velocities and temperatures. For a 10 km
G. The neutrals, ions and electrons can thus possess independent velocities and temperatures. For a 10 km  shock and
shock and 
 G, they calculate a maximum neutral gas temperature of 925 K and a maximum ion-neutral drift velocity of 4.6 km
G, they calculate a maximum neutral gas temperature of 925 K and a maximum ion-neutral drift velocity of 4.6 km  over a length scale in which the ions and neutrals are decoupled,
over a length scale in which the ions and neutrals are decoupled,  cm. Despite the much lower temperatures post-shock, the differential motion of the ions and neutrals is sufficient to overcome the endothermicity of the
cm. Despite the much lower temperatures post-shock, the differential motion of the ions and neutrals is sufficient to overcome the endothermicity of the  reaction. The large length scale over which decoupling occurs leads to a
reaction. The large length scale over which decoupling occurs leads to a  column density of
column density of 
 , that is, the inclusion of magnetic fields on the shock structure drives the chemistry more efficiently and leads to column densities much enhanced over the non-MHD models and close to those observed, at least for those species for which endothermic ion-neutral reactions control their chemistry.
, that is, the inclusion of magnetic fields on the shock structure drives the chemistry more efficiently and leads to column densities much enhanced over the non-MHD models and close to those observed, at least for those species for which endothermic ion-neutral reactions control their chemistry.
In the real world of course, there are many geometries and other parameters, including time-dependence of the underlying source of energy or momentum, to consider but the overall conclusion remains. MHD shocks can induce an ion-neutral drift over an extended region of space and the relative velocity of colliding partners can drive endoergic reactions, overcome activation energy barriers and result in enhanced abundances of molecules that would otherwise be difficult to produce.
3. Astrochemistry in other environments
Although the chemistry discussed to date has concentrated on those regions associated with star formation, molecules also play an important role in other astrophysical regions in which gas-phase, plasma reactions can be important. In this section, we discuss two such regions, the early universe in the first 1000 Myr after the Big Bang and in the circumstellar envelopes of old, dying stars.
3.1. Early universe
The conventional picture of the origin of the Universe starts with the Big Bang out of which space and time, and the elements which form the material Universe, were created. In this expanding and cooling Universe, collisions of protons, neutrons and electrons were fast enough to create only the light elements—H, He, Li and their isotopes most importantly—before the expansion effectively 'froze' nucleosynthesis. Astronomers measure distances in the Universe through the cosmological redshift, z, due to the expansion of the Universe, as:

where  and
and  are the wavelengths of the emitted and observed light. The relationship between redshift and the age of the Universe at the point the light was emitted depends on the details of the cosmological model that one adopts but is proportional to
are the wavelengths of the emitted and observed light. The relationship between redshift and the age of the Universe at the point the light was emitted depends on the details of the cosmological model that one adopts but is proportional to  for large z. As the Universe expanded, the initial plasma cooled and recombined in the so-called 'recombination era', which began at a redshift, z, of about 800–1000, or around five hundred thousand years after the Big Bang, when the gas temperature had fallen to
for large z. As the Universe expanded, the initial plasma cooled and recombined in the so-called 'recombination era', which began at a redshift, z, of about 800–1000, or around five hundred thousand years after the Big Bang, when the gas temperature had fallen to  8000 K, and continued to its re-ionization due to the first generation of massive stars at
8000 K, and continued to its re-ionization due to the first generation of massive stars at  , at an age of about 250 Myr. In this era, molecular hydrogen formed through gas-phase processes involving
, at an age of about 250 Myr. In this era, molecular hydrogen formed through gas-phase processes involving  :
:
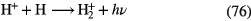
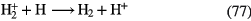
and  :
:
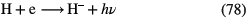
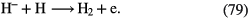
These two routes tend to occur at different redshifts because the  ion is more easily destroyed by photons than
ion is more easily destroyed by photons than  and is not abundant until the Universe has cooled sufficiently to prevent the formation of high-energy photons. Formation of
and is not abundant until the Universe has cooled sufficiently to prevent the formation of high-energy photons. Formation of  via
via  peaks at
peaks at  and via
and via  at
at  . These specific reactions have slow, rate-limiting steps so that the amount of
. These specific reactions have slow, rate-limiting steps so that the amount of  formed is small, with a fractional abundance of
formed is small, with a fractional abundance of  . Other molecules also form, notably HD and LiH, in reactions such as:
. Other molecules also form, notably HD and LiH, in reactions such as:
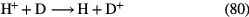
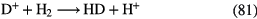
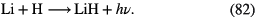
The abundance of such molecules remains small but because both possess a permanent electric dipole moment they are able to contribute to the cooling of the gas.  is, however, the dominant coolant and the gas cools from
is, however, the dominant coolant and the gas cools from  4000 K to
4000 K to  200 K. This large decrease in temperature causes a reduction in the internal pressure of the gas and, despite the fact that the Universe itself is expanding, this reduction allows gravitational collapse to proceed and the first stars and galaxies to form. An excellent review of the chemistry in pre-galactic gas and in the mini-haloes of proto-galaxies can be found in Glover (2011). He shows that, since the density and temperature of the Universe is changing with time, the detailed cooling of the gas and the masses of the structures which form are very sensitive to the particular values of the rate coefficients over a wide range of temperature. An extensive review of the chemistry of the light atoms created in the Big Bang is given by Galli and Palla (2013).
200 K. This large decrease in temperature causes a reduction in the internal pressure of the gas and, despite the fact that the Universe itself is expanding, this reduction allows gravitational collapse to proceed and the first stars and galaxies to form. An excellent review of the chemistry in pre-galactic gas and in the mini-haloes of proto-galaxies can be found in Glover (2011). He shows that, since the density and temperature of the Universe is changing with time, the detailed cooling of the gas and the masses of the structures which form are very sensitive to the particular values of the rate coefficients over a wide range of temperature. An extensive review of the chemistry of the light atoms created in the Big Bang is given by Galli and Palla (2013).
The first generation of stars in the Universe is then able to produce the heavier elements—C, N, O, etc—through stellar nucleosynthesis and return these elements to the interstellar medium through supernova explosions and stellar winds. Although we do not detect the first molecules to form, HD and LiH, the molecule CO has been detected in several galaxies out to redshift of 6 (Wang et al 2010). The record is in the quasar SDSS J1148 + 5251 at  , some 890 Myr after the Big Bang (Walter et al 2003). This object has over
, some 890 Myr after the Big Bang (Walter et al 2003). This object has over  of molecular gas and is forming stars at a rate of 3000
of molecular gas and is forming stars at a rate of 3000 
 , roughly 1000 times the value in the Milky Way.
, roughly 1000 times the value in the Milky Way.
3.2. Circumstellar envelopes
Molecules also form very efficiently in cool stars. Stars with masses in the range 1–8  end their lives through losing mass in a stellar wind to the interstellar medium—higher mass stars become supernovae and return material explosively. At the end of their nuclear-burning phase, the stars are very large, with photospheric radii on the order of 200–300
end their lives through losing mass in a stellar wind to the interstellar medium—higher mass stars become supernovae and return material explosively. At the end of their nuclear-burning phase, the stars are very large, with photospheric radii on the order of 200–300  , and relatively cool with effective temperatures of 2000–3000 K, and are known as asymptotic giant branch (AGB) stars. These winds, which typically have velocities of 10–25 km
, and relatively cool with effective temperatures of 2000–3000 K, and are known as asymptotic giant branch (AGB) stars. These winds, which typically have velocities of 10–25 km  , eventually, over a few 10 000 yrs, remove the outer layers of the stellar atmosphere to form a planetary nebula with a central, hot, white dwarf star.
, eventually, over a few 10 000 yrs, remove the outer layers of the stellar atmosphere to form a planetary nebula with a central, hot, white dwarf star.
The nature of the molecules and the chemistry that occurs in the circumstellar envelopes (CSEs) formed by the mass loss depends on the overall carbon-to-oxygen, C/O, ratio and on the properties of three main radial zones around the central star. These CSEs can be very rich in molecules, particularly those which are carbon-rich: the archetype of these, the star IRC+10216 or CW Leo, with a mass loss rate of 2 

 and an expansion velocity of 14.5 km
and an expansion velocity of 14.5 km  , contains over 80 molecules including many carbon-chain species, as found in dark clouds, and several metal halides including NaCl, KCl and AlCl, molecules yet to be detected in interstellar clouds.
, contains over 80 molecules including many carbon-chain species, as found in dark clouds, and several metal halides including NaCl, KCl and AlCl, molecules yet to be detected in interstellar clouds.
The chemistry occurring in CSEs can be considered to proceed in three regions delineated by radial distance from the central star, which has a photospheric (stellar) radius,  , of a few
, of a few  cm. It should be noted that stellar UV photons are unimportant in the chemistry since the stars are cool.
cm. It should be noted that stellar UV photons are unimportant in the chemistry since the stars are cool.
3.2.1. Photospheric chemistry.
At the high densities, more than 
 , and temperatures experienced at the photosphere, typically 2500–3500 K, too hot for dust grains to survive, molecules are formed in local thermodynamic equilibrium (LTE). Three-body collisions among neutral species dominate—one of the few astrochemical situations where this is the case—and molecular abundances are determined by minimising the Gibbs free energy:
, and temperatures experienced at the photosphere, typically 2500–3500 K, too hot for dust grains to survive, molecules are formed in local thermodynamic equilibrium (LTE). Three-body collisions among neutral species dominate—one of the few astrochemical situations where this is the case—and molecular abundances are determined by minimising the Gibbs free energy:

where  is the number of moles of species i and
is the number of moles of species i and  its chemical potential:
its chemical potential:
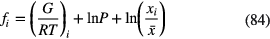
with  the Gibbs free energy of species i, P the total pressure of the gas, and
the Gibbs free energy of species i, P the total pressure of the gas, and  . LTE preferentially forms molecules with high dissociation energies, in particular CO is the most abundant molecule after
. LTE preferentially forms molecules with high dissociation energies, in particular CO is the most abundant molecule after  for conditions typical of C-rich and O-rich AGB stars. In C-rich stars, essentially all available oxygen is tied up in CO (bond energy 11.2 eV). The excess carbon ends up in abundant species such as
for conditions typical of C-rich and O-rich AGB stars. In C-rich stars, essentially all available oxygen is tied up in CO (bond energy 11.2 eV). The excess carbon ends up in abundant species such as  , HCN and CS, with nitrogen partitioned into molecules such as
, HCN and CS, with nitrogen partitioned into molecules such as  and HCN. Observations of all these species, with the exception of
and HCN. Observations of all these species, with the exception of  , can be made close to the photosphere through infrared absorption and emission spectroscopy. In O-rich stars, CO,
, can be made close to the photosphere through infrared absorption and emission spectroscopy. In O-rich stars, CO,  O and SiO are the most abundant O-bearing species.
O and SiO are the most abundant O-bearing species.
In modelling the chemistry of AGB stars, it is often the case that LTE calculations are used to set the abundances of 'parent' species which then flow out into the outer regions of the CSE. There are, however, two major processes which perturb the LTE abundances.
3.2.2. Pulsations and dust formation.
The inner regions of the CSE are not stable structures since AGB stars pulsate on time-scales of 1–3 years, typically. These sub-sonic pulsations generated in the interior of the star drive compression waves through the atmosphere steepening into shocks. Such shocks lose energy either radiatively, when the density is high and the shocks can be treated as isothermal, or by adiabatic expansion when the density is low. Detailed models of these shocks have been made by Bowen (1988) who showed that strong shocks occur on a cyclic basis and create an extended atmosphere in which the shock velocity decreases as the gas expands. A particular parcel of gas which receives an outward impulse roughly follows a ballistic trajectory before falling back towards the stellar surface under the influence of gravity. If it experiences a second shock before it returns to its initial position, it attains a net outward momentum and can drive mass loss.
Willacy and Cherchneff (1998) studied the chemistry in the inner 5  induced by these periodic shock waves on a molecular gas whose initial composition is determined by LTE. The chemistry is dominated by neutral–neutral reactions which, if they can occur faster than the dynamical time-scales, can alter LTE abundances dramatically. Key reactants at these high temperatures and densities are atomic hydrogen and O atoms formed by collisional dissociation of
induced by these periodic shock waves on a molecular gas whose initial composition is determined by LTE. The chemistry is dominated by neutral–neutral reactions which, if they can occur faster than the dynamical time-scales, can alter LTE abundances dramatically. Key reactants at these high temperatures and densities are atomic hydrogen and O atoms formed by collisional dissociation of  and CO. In C-rich stars, these O atoms can react with
and CO. In C-rich stars, these O atoms can react with  to form OH and
to form OH and  O, while atomic silicon, the dominant form of the element in LTE, reacts with OH to form SiO, increasing the latter abundance by more than a factor of 100 compared to its LTE value and giving closer agreement with the abundance observed close to the photosphere.
O, while atomic silicon, the dominant form of the element in LTE, reacts with OH to form SiO, increasing the latter abundance by more than a factor of 100 compared to its LTE value and giving closer agreement with the abundance observed close to the photosphere.
Shock chemistry, driven by underlying pulsations, does seem to be required to explain the relatively high abundances of O-bearing molecules, including OH,  O and
O and  CO, detected in IRC+10216 in recent years. Cherchneff (2012) gives an excellent summary of the physics and chemistry of this inner region and shows that, in addition to O-bearing molecules, shock chemistry can produce large abundances of chlorides such as HCl, AlCl and NaCl, as also observed.
CO, detected in IRC+10216 in recent years. Cherchneff (2012) gives an excellent summary of the physics and chemistry of this inner region and shows that, in addition to O-bearing molecules, shock chemistry can produce large abundances of chlorides such as HCl, AlCl and NaCl, as also observed.
AGB stars are also the major producer, perhaps up to 80%, of dust particles in the Galaxy. Infrared observations show that the dust composition is either amorphous carbon in C-rich stars or silicates in O-rich stars. The formation of these are not well understood although it is clear that it occurs within a few stellar radii. Initial research on dust formation in carbon stars invoked classical nucleation theory in which solids condense out of a cooling gas once the partial pressure of a particular species exceeds its vapour pressure. Nucleation theory describes growth from gas-phase monomers and identifies a critical cluster size above which growth by addition of a monomer is energetically favoured. This approach has had only limited success in its application to AGB stars. For O-rich stars, there is no monomer out of which the observed silicates can grow whilst in C-rich stars, there are kinetic bottlenecks in the formation of the first few ring molecules, and in both types the short dynamical time-scales can mitigate against grain formation. Pulsations again seem to be critical. The levitation of material in the atmosphere, the density increase caused by the propagation of shock waves, and the fast cooling post-shock, have been included in a kinetic description of the chemistry in C-rich stars (Cherchneff (2012) and references therein). In this model for IRC+10216, she showed that the abundance of benzene peaked at a fractional abundance of  at around 3
at around 3  . Assuming that benzene is converted to coronene,
. Assuming that benzene is converted to coronene,  , through a series of H-abstraction, acetylene-addition reactions, and that this is a proxy for the dust mass, she showed that dust masses consistent with those observed can be achieved.
, through a series of H-abstraction, acetylene-addition reactions, and that this is a proxy for the dust mass, she showed that dust masses consistent with those observed can be achieved.
In O-rich stars, the dominant dust component is amorphous silicate with evidence also for crystalline silicates. The most abundant oxide in the gas after CO in the dust-forming zone is SiO which has a condensation temperature of around 600 K, much lower than the observed dust temperature, around 1000 K. It is thus likely to be the more refractory oxides, such as TiO,  , AlO and
, AlO and  , which form the first condensates on which further grain growth can occur. Corundum,
, which form the first condensates on which further grain growth can occur. Corundum,  , is the most abundant Al-containing molecule and can condense out of the gas below 1000 K (Sharp and Huebner 1990)—some 90% of all pre-solar oxide grains found in meteorites contain corundum that condensed in O-rich AGB stars. LTE conditions, however, are unlikely to hold given stellar pulsations and the generation of periodic shock waves but the identification of the detailed chemical reactions that form the smallest molecular clusters is still elusive. Kinetic models of the chemistry are difficult to describe although Gail and Sedlmayr (1998) have shown that solid
, is the most abundant Al-containing molecule and can condense out of the gas below 1000 K (Sharp and Huebner 1990)—some 90% of all pre-solar oxide grains found in meteorites contain corundum that condensed in O-rich AGB stars. LTE conditions, however, are unlikely to hold given stellar pulsations and the generation of periodic shock waves but the identification of the detailed chemical reactions that form the smallest molecular clusters is still elusive. Kinetic models of the chemistry are difficult to describe although Gail and Sedlmayr (1998) have shown that solid  may provide the seed nuclei on which silicates condense—the low abundance of Ti compared to Si and Mg, however, may be problematic in this scenario. Goumans and Bromley (2012) have investigated the thermodynamics of small cluster formation in a 1000 K gas of
may provide the seed nuclei on which silicates condense—the low abundance of Ti compared to Si and Mg, however, may be problematic in this scenario. Goumans and Bromley (2012) have investigated the thermodynamics of small cluster formation in a 1000 K gas of  , SiO, Mg and
, SiO, Mg and  O and find that the homomolecular nucleation of SiO stops at the dimer, whereas Mg can be incorporated exothermically into silicon oxides when the number of oxygen atoms is larger than that of the metal atoms.
O and find that the homomolecular nucleation of SiO stops at the dimer, whereas Mg can be incorporated exothermically into silicon oxides when the number of oxygen atoms is larger than that of the metal atoms.
Once formed, dust grains become the most important absorber of stellar photons and the transfer of photon momentum to the dust, and subsequently to the gas through collisions, initiates a rapid acceleration of the gas and drives the mass-loss process. As the gas and dust flows outwards, collisions between them are possible although a comparison between the gas-grain collision time and the expansion time shows that this is important only within  cm for typical conditions. There has been, as yet, little attempt to study the gas-dust interaction in this zone, where both the gas and the grains are hot, but there is evidence that molecule formation mediated by this interaction does occur. For example, 10
cm for typical conditions. There has been, as yet, little attempt to study the gas-dust interaction in this zone, where both the gas and the grains are hot, but there is evidence that molecule formation mediated by this interaction does occur. For example, 10  m observations of silane,
m observations of silane,  , in IRC+10216 (Keady and Ridgway 1993) shows that it is formed at radii beyond 40
, in IRC+10216 (Keady and Ridgway 1993) shows that it is formed at radii beyond 40  —similar results hold for
—similar results hold for  and
and  . The increased abundances of these and other hydrides in this region may imply, as it does in the case of hot molecular cores, that molecules are being formed on the surfaces of dust grains through hydrogenation of atoms, although other explanations are possible.
. The increased abundances of these and other hydrides in this region may imply, as it does in the case of hot molecular cores, that molecules are being formed on the surfaces of dust grains through hydrogenation of atoms, although other explanations are possible.
Consider  O which was detected in IRC+10216 through submillimetre satellite observations (Melnick et al 2001). This observation was a surprise since neither the LTE models for the inner chemistry nor the models of the outer CSE predicted water. This observation, which was followed by observations of OH and
O which was detected in IRC+10216 through submillimetre satellite observations (Melnick et al 2001). This observation was a surprise since neither the LTE models for the inner chemistry nor the models of the outer CSE predicted water. This observation, which was followed by observations of OH and  CO in the same star, led to suggestions that water was formed by the evaporation of icy comets orbiting within the CSE (Melnick et al 2001), or that it could be formed in the dust growth zone by the Fischer–Tropsch mechanism on the surface of iron grains (Willacy 2004). Subsequent observations of high-excitation transitions using the Herschel Space Observatory (Decin et al 2010) showed unequivocally that water is warm, several hundred K, and confined to the inner envelope. Cherchneff (2011) argued that this is consistent with recent shock models, although several of the key rate coefficients are unknown or highly uncertain. Agúndez et al (2010) used the observed clumpy nature of the CSE to postulate that external UV photons can penetrate deep into the inner regions of the envelope and drive the photodissociation of molecules such as SiO and
CO in the same star, led to suggestions that water was formed by the evaporation of icy comets orbiting within the CSE (Melnick et al 2001), or that it could be formed in the dust growth zone by the Fischer–Tropsch mechanism on the surface of iron grains (Willacy 2004). Subsequent observations of high-excitation transitions using the Herschel Space Observatory (Decin et al 2010) showed unequivocally that water is warm, several hundred K, and confined to the inner envelope. Cherchneff (2011) argued that this is consistent with recent shock models, although several of the key rate coefficients are unknown or highly uncertain. Agúndez et al (2010) used the observed clumpy nature of the CSE to postulate that external UV photons can penetrate deep into the inner regions of the envelope and drive the photodissociation of molecules such as SiO and  CO which release O atoms into the gas phase—
CO which release O atoms into the gas phase— CO is optically thick to dissociating photons. The O atoms then react with
CO is optically thick to dissociating photons. The O atoms then react with  in the warm gas to form OH and water.
in the warm gas to form OH and water.
3.2.3. Photochemistry in the outer envelope.
In the very outer reaches of the circumstellar envelope, external UV photons can disrupt the chemistry, giving rise a rich soup of radicals and ions that can react further. An interesting outcome in some CSEs, as discussed below, is that the chemistry produces relatively high abundances of anions, one of the few regions in the ISM in which they are formed in observable quantities.
To understand the underlying physical properties and chemistry of the gas, let us assume that gas and dust flow out from the star in a steady, uniform, spherically symmetric flow at terminal velocity v and mass-loss rate  and which is irradiated by external UV photons from the interstellar radiation field. In this case, we can use conservation of mass to derive the
and which is irradiated by external UV photons from the interstellar radiation field. In this case, we can use conservation of mass to derive the  abundance as a function of radius. We can then write the radial number density of
abundance as a function of radius. We can then write the radial number density of  ,
,  , the radial column density of
, the radial column density of  from radial distance r to infinity, N
from radial distance r to infinity, N , and the radial extinction in the UV due to dust,
, and the radial extinction in the UV due to dust,  as:
as:
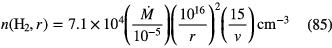

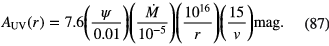
Here the mass-loss rate is measured in 
 , the radial distance in cm, the terminal velocity in km
, the radial distance in cm, the terminal velocity in km  and
and  is the dust-to-gas mass ratio, where 0.01 is its typical value in the interstellar medium. It is likely to vary from object to object in AGB stars, with a typical value of perhaps 0.003. The temperature profile of the gas is determined by adiabatic expansion and molecular line and dust thermal cooling but can be well approximated by a power-law distribution
is the dust-to-gas mass ratio, where 0.01 is its typical value in the interstellar medium. It is likely to vary from object to object in AGB stars, with a typical value of perhaps 0.003. The temperature profile of the gas is determined by adiabatic expansion and molecular line and dust thermal cooling but can be well approximated by a power-law distribution  , with
, with  around 0.6–0.7. At an injection radius of 2
around 0.6–0.7. At an injection radius of 2  cm, the
cm, the  density is about 3
density is about 3 
 , the temperature is 220 K and the radial visual extinction,
, the temperature is 220 K and the radial visual extinction,  , about 7 mag, for parameters typical of those in IRC+10216 (McElroy et al 2013).
, about 7 mag, for parameters typical of those in IRC+10216 (McElroy et al 2013).
One sees from these equations, that since external UV photons begin to interact with outflowing parent species once the UV extinction falls below about 10 mags, that is, at a radius of around  cm for parameters typical of AGB stars. One should note, however, that three of the major parent species, namely
cm for parameters typical of AGB stars. One should note, however, that three of the major parent species, namely  ,
,  and CO experience self- and mutual-shielding against photodestruction. Indeed the
and CO experience self- and mutual-shielding against photodestruction. Indeed the  photodissociation rate is negligible to distances typically in excess of 1 parsec (
photodissociation rate is negligible to distances typically in excess of 1 parsec ( cm) from the star. In C-rich AGB stars, the most important parents for driving chemistry are
cm) from the star. In C-rich AGB stars, the most important parents for driving chemistry are  and HCN. The photodissociation of HCN gives rise to CN which is itself photodissociated to N and C atoms, with the latter photoionized in the very outer envelope. Thus, external photons cause the formation of molecular shells, whose radial position depend on the underlying flow properties and the photon flux. Acetylene can be both ionized, to form
and HCN. The photodissociation of HCN gives rise to CN which is itself photodissociated to N and C atoms, with the latter photoionized in the very outer envelope. Thus, external photons cause the formation of molecular shells, whose radial position depend on the underlying flow properties and the photon flux. Acetylene can be both ionized, to form  , and dissociated, to form
, and dissociated, to form  H,
H,  , C and
, C and  , sequentially. The result is that one finds a region in the CSE where abundant photons, radicals and ions co-exist at relatively high density,
, sequentially. The result is that one finds a region in the CSE where abundant photons, radicals and ions co-exist at relatively high density, 
 , and cold temperatures, 100–10 K, a situation that is relatively rare in astronomy. Collisions between these reactive species then give rise to the molecular complexity observed in C-rich CSEs through reactions described in section 2.2.
, and cold temperatures, 100–10 K, a situation that is relatively rare in astronomy. Collisions between these reactive species then give rise to the molecular complexity observed in C-rich CSEs through reactions described in section 2.2.
One of the interesting aspects of the chemistry is its propensity to form carbon-chain molecules and anions— ,
,  ,
,  ,
,  ,
,  ,
,  have been detected in IRC+10216—in relatively high abundance; indeed the total anion abundance is found to exceed that of free electrons in some parts of the outer envelope (Millar et al 2007, Cordiner and Millar 2009). Figure 16 from McElroy et al (2013) shows the radial distributions of a number of important linear hydrocarbon molecules and anions. We note that in IRC+10216, the observed ratios for
have been detected in IRC+10216—in relatively high abundance; indeed the total anion abundance is found to exceed that of free electrons in some parts of the outer envelope (Millar et al 2007, Cordiner and Millar 2009). Figure 16 from McElroy et al (2013) shows the radial distributions of a number of important linear hydrocarbon molecules and anions. We note that in IRC+10216, the observed ratios for  /
/ H and
H and  /
/ H are 0.09 and 0.26, respectively (Kasai et al 2007, Remijan et al 2007).
H are 0.09 and 0.26, respectively (Kasai et al 2007, Remijan et al 2007).
Figure 16. Top left: plot of the fractional abundances, relative to  , of cyanopolyynes as a function of envelope radius using the Rate12 model (solid lines) compared with the results from Cordiner and Millar (2009) (dotted lines). Top right: Plot of fractional abundances of polyynes as a function of envelope radius for the Rate12 model including anion chemistry (solid lines) and excluding anion chemistry (dashed lines). Bottom left: plot of fractional abundances of polyyne anions as a function of envelope radius. Bottom right: comparison of the fractional abundances of various cations and electrons, including anion chemistry (solid lines) and excluding anion chemistry (dashed lines). The
, of cyanopolyynes as a function of envelope radius using the Rate12 model (solid lines) compared with the results from Cordiner and Millar (2009) (dotted lines). Top right: Plot of fractional abundances of polyynes as a function of envelope radius for the Rate12 model including anion chemistry (solid lines) and excluding anion chemistry (dashed lines). Bottom left: plot of fractional abundances of polyyne anions as a function of envelope radius. Bottom right: comparison of the fractional abundances of various cations and electrons, including anion chemistry (solid lines) and excluding anion chemistry (dashed lines). The  fractional abundance for the 'anions included' model is shown, for reference, with a dot-dashed line (McElroy et al (2013) © ESO.)
fractional abundance for the 'anions included' model is shown, for reference, with a dot-dashed line (McElroy et al (2013) © ESO.)
Download figure:
Standard image High-resolution imageThe cyanopolyynes,  N are formed via neutral-neutral reactions between CN and the polyynes:
N are formed via neutral-neutral reactions between CN and the polyynes:
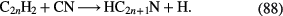
Large carbon-chain molecules often possess large electron affinities and can undergo radiative attachment with electrons, with rate coefficients that generally increase with size of the neutral (Herbst and Osamura 2008). Thus  , because of the relatively large abundance of
, because of the relatively large abundance of  H and fast radiative attachment of electrons to
H and fast radiative attachment of electrons to  H, is the dominant anion in the outer CSE. The formation of the cyanopolyyne anions occurs by radiative attachment as well as through reactions between N atoms and anions (Eichelberger et al 2007):
H, is the dominant anion in the outer CSE. The formation of the cyanopolyyne anions occurs by radiative attachment as well as through reactions between N atoms and anions (Eichelberger et al 2007):
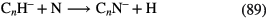
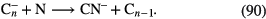
Figure 16, top right panel, shows the interesting result that the presence of these large hydrocarbon anions act to enhance the formation of large hydrocarbon chain molecules in the outer CSE as noted previously for dark clouds (Walsh et al 2009). A unique impact on the CSE chemistry, however, is that the free electron abundance can be depressed by an order of magnitude below the anion abundance in the range 0.3–1.0  cm in figure 16. This reduction in electron abundance leads to the decreased importance of dissociative electron recombination as a loss mechanism for cations, see the lower right panel in figure 16, with cations increasing in abundance by factors of 10–1000. The lower right panel also shows the radial abundance of
cm in figure 16. This reduction in electron abundance leads to the decreased importance of dissociative electron recombination as a loss mechanism for cations, see the lower right panel in figure 16, with cations increasing in abundance by factors of 10–1000. The lower right panel also shows the radial abundance of  which is the dominant carrier of negative charge in the region 3–6
which is the dominant carrier of negative charge in the region 3–6  cm.
cm.
The chemical reactions that synthesise molecules in the CSE are thus identical to those occurring in cold dark clouds. In the latter, however, the chemistry is acting to transform atoms to molecules, whereas in the envelopes of AGB stars, the chemistry acts to transform stable molecules formed in and near the photosphere to atoms and atomic ions which return to the interstellar medium to begin the process of cloud formation and collapse, star and planet formation, stellar evolution and star death, and another cycle in the history of chemistry in the Galaxy.
4. Summary
We have seen that chemistry controls many aspects of the evolution of the Universe, in particular the formation of stars, and that observations of molecular line transitions are powerful probes of physics, in the determination of number densities, temperatures and gas dynamics. Much of the chemistry occurs at temperatures and pressures much less than those experienced in terrestrial laboratories and over time scales of up to one million years. Nevertheless, laboratory experiments are urgently needed to understand important processes and provide key reaction rate coefficients. Among these are dissociative recombination of protonated molecules, particularly large organic molecules, with electrons. Here, the branching ratios are often more important to know than the overall rate coefficient. As an example, protonated methanol produces methanol in only 3% of its dissociative recombinations with electrons (Geppert et al 2006) rather than the 50% normally assumed in models at that time. This low branching ratio is not sufficient to produce methanol at its observed abundances in molecular clouds (Wirström et al 2011). It indicates, rather, that methanol is produced through the hydrogenation of CO in icy grain mantles, consistent with the detection of  OH (Parise et al 2004) and laboratory experiments on the surface chemistry of CO with D and H atoms (Watanabe et al 2004, Hidaka et al 2009). Other protonated species which have very small branching ratios to their parent neutral include protonated ethanol and protonated dimethyl ether (Hamberg et al (2010a), 2010b) but not all dissociative recombinations of protonated COMs are inefficient, propionitrile is produced in 43% of recombinations (Vigren et al 2010). Laboratory studies of the rate coefficients and branching ratios of the dissociative recombination of protonated COMs and other large molecular ions, for example PAH cations, remain an important topic in astrochemistry.
OH (Parise et al 2004) and laboratory experiments on the surface chemistry of CO with D and H atoms (Watanabe et al 2004, Hidaka et al 2009). Other protonated species which have very small branching ratios to their parent neutral include protonated ethanol and protonated dimethyl ether (Hamberg et al (2010a), 2010b) but not all dissociative recombinations of protonated COMs are inefficient, propionitrile is produced in 43% of recombinations (Vigren et al 2010). Laboratory studies of the rate coefficients and branching ratios of the dissociative recombination of protonated COMs and other large molecular ions, for example PAH cations, remain an important topic in astrochemistry.
The recent detection of anions and the conclusion that they could be more abundant than free electrons in some regions (section 3.2.3), implies that it will also be important to study cation–anion recombinations. A number of processes involving anions still remain relatively unexplored at low energies in the laboratory. These include processes such as radiative attachment, associative electron detachment and mutual neutralisation. In cold dark molecular clouds, it is likely that the population of PAH-like molecules (section 1.2) are negatively charged; here again, laboratory studies of their reactions with atoms, radicals, neutral molecules and cations are lacking, although Bierbaum and colleagues have made some important measurements on the reactivities of PAH cations and anions (Demarais et al 2012, 2014). Such reactions may also have particular relevance to astrobiology.
The interaction of UV photons and cosmic ray particles with the gas means that chemical reactions are predominantly ion-neutral in nature, such processes contributing to the enormous isotope enhancements that are detected in deuterated species. The chemistry of the interstellar medium is, however, not restricted to the gas phase and reactions in and on the icy mantles of grains, although very poorly understood at present, do seem to be critical in the formation of complex organic molecules in star-forming regions. Significant work needs to be done to better understand the mechanisms by which photons and high-energy electrons and cosmic-ray protons interact with water ice and the mixed and layered ices thought to be present in the ISM. There remains uncertainty over the competition between dissociation of ice molecules, which can create reactive radicals and ions in the ice, the reactive pathways that ions, in particular, may follow and the desorption of these radicals and their parents. Binding energies and diffusion barriers control the mobility and reactivity of material in the ice and are a particular source of uncertainty given the exponential dependence of relevant time scales on these parameters. Many models of grain surface chemistry assume the same energetics and outcomes as gas-phase chemistry—the dissociative recombination of molecular ions on the surfaces of negatively charged grains is one such example—and must surely be a source of great uncertainty in modelled outcomes. Finally, we note that the characteristics of the grains themselves must play a key role in the chemistry through their composition, porosity, size distribution, for example. It is clear from current experiments that the ice chemistry is complex although still ill-defined in a global sense. We may have some ideas and a reasonable degree of certainty as to how simple species such as water and methanol form through hydrogenation of O and CO respectively, but we have much less confidence in describing how complex molecules form on grains. Current chemical kinetic models contain several thousand gas-phase reactions and only a few hundred surface reactions. It is likely that chemistry in the bulk ice needs to be considered more fully given that energetic interactions happen here rather than in the surface layer and that many hundred, if not thousands, of ice reactions need to be included—a very challenging task.
The challenge, however, may have great rewards. Evidence from the laboratory, from the composition of meteorites and from the Stardust comet return mission indicate that complex molecules, including many amino acids and nucleobases, can be readily formed under conditions similar to those present in interstellar clouds. Can these pre-biotic molecules help the emergence of life in our own and other solar systems? A continuing close collaboration between astronomers, chemists and physicists—and biologists—may provide the answer to this within the lifetime of our current graduate students.
Acknowledgments
Molecular Astrophysics at QUB is supported by a grant from the Science and Technology Facilities Council. I am very grateful to the referees whose careful reading and comments improved this article.