Abstract
Carbon nanowalls (CNWs) are composed of stacks of planar graphene layers with open edges that grow almost vertically on a substrate. Their morphology makes them a promising material for field emission, batteries, light absorbers and enhanced detectors for electrochemical and gas sensors. However, three main challenges prevent the fast development of CNWs: the synthesis is energetically demanding, poorly transferable to suitable substrates, and the growth mechanism is not understood. Here, we present a simple method to grow carbon nanowall-like flowers on multilayer graphene through fullerenic particles using thermal CVD and copper. The hydrophobicity of the fabricated hybrid material facilitates its transfer to any substrate. Our findings can boost the understanding of the physical properties and the practical applicability of CNWs. At the same time, our work is a concrete example of the role of multilayer graphene as a platform to one-step synthesis of new transferable graphenic materials.
Export citation and abstract BibTeX RIS
1. Introduction
Three-dimensional graphitic nanostructures with sharp edges like multilayer graphene, carbon nanotubes, nanoribbons, and porous carbon have attracted considerable attention. Since their edges play the role of large defects within the finite flat hexagonal network, they play a predominant role in enhancing electronic, chemical, magnetic, and optical properties of materials [1, 2]. The incidental discovery of carbon nanowalls (CNWs) [3, 4] has opened up new perspectives to fabricate nanosized carbon with a high density of edges. Their fringes are composed of stacking a number of graphene sheets with different interlayer distances, standing almost perpendicularly to the substrate. Because CNWs exhibit open edges, they are better suited as a material for chemical and physical manipulations than nanotubes, nanoribbons, or graphite which has large smooth hexagonal basal planes that are less reactive compared to its edges. Moreover, carbon edges have demonstrated great ability to anchor or intercalate ions, molecules, or nanoparticles, which increases the electrochemical performance of carbon materials [5–7]. Furthermore, the high density of atomic edges was explored as having potential for electron field emission emitters [8]. To date, many morphologies and structures of CNWs have been reported. For instance, structures such as isolated sheets, roses, maze-like walls, highly branched types, and dense porous films have been fabricated [3, 7]. Plasma enhanced chemical vapour deposition techniques are mainly used to synthesize CNWs [7, 9]. In contrast to many carbonaceous nanostructures, CNWs have been grown on both metallic and non-metallic substrates [4, 10, 11]. Therefore, it is concluded that a catalyst is not necessary for the synthesis of CNWs and the substrate can be chosen based on application. Even though the discovery of CNWs was revealed before graphene and they have been intensively studied over the last decade, their full potential is not widely investigated due to the following challenges: (i) it is not possible to transfer CNWs after growth to other substrates and all the chemical and/or physical processes have been achieved on the substrate of growth. (ii) The use of highly energetic systems to grow CNWs requires rigorous control of the process. Otherwise, undesirable side reactions occur leading to the formation of other carbonaceous structures which prevents having the large scale, clean, and homogeneous material that is essential for high performance applications. (iii) The mechanism of CNWs growth is still not understood, and the conditions that govern the nucleation process are unknown.
The present work reports a simple method of growing CNW-like flowers (CNW-LFs) using a simple thermal chemical vapour deposition (CVD) method. The use of copper as a catalyst can be understood through the following three steps. During the first step only multilayer graphene is spread out over copper while non-graphitizing carbon is formed in the second step, mainly characterized by crumpled, curled, and disordered graphene sheets. The blooming of CNW-LFs takes place on areas covered by non-graphitizing carbons during the last step. The nucleation of CNW-LFs is found to be ignited through fullerenic particles under high pressure of hydrogen as discussed in the main text below. The graphenic material based on graphene derivatives is hydrophobic which permits its straightforward transfer onto other substrates after removing the copper by wet etching.
2. Methods
Both camphor and tetraacetylethane were used as precursors to carry out the growth of CNW-LFs. Synthetic camphor was purchased from Sigma Aldrich (CAS number 0000076222) while tetraacetylethane was synthesized following the procedures described in the literature [12] with small modifications (supplementary data stacks.iop.org/NANO/27/175603/mmedia). The thermal CVD process was used under high purity of hydrogen and the solid feedstock was introduced at the inlet of the chamber. While the tetraacetylethane was placed upstream in a quartz tube and 20 cm away from the chamber, camphor powder was contained in a Pyrex tube separated from the chamber by an isolating valve. Before the copper foils (25 μm thickness) were introduced to the quartz tube they were dumped into acetic acid to remove the thin protective oxide layer as well as surface damage. Once the solution became bluish the foils were then removed, washed several times by deionized water, and loaded into the chamber. A post-annealing treatment was performed at 950 °C for 20 min under 1 Torr of hydrogen pressure to improve the crystallinity of copper. Then, the pressure was increased up to 10 Torr for tetraacetylethane and 400 Torr for camphor. 2 g of camphor was enough to ensure full coverage of CNW-LFs over copper while 4 g of tetraacetylethane was needed to reproduce same results. Moreover, to draw tetraacetylethane inside the chamber, the precursor was heated up to 180 °C. In contrast, camphor was drawn without thermal annealing since it is a volatile solid. Once the precursor was used up, hydrogen flow was decreased to 1 Torr and maintained during the cool-down of the chamber to room temperature, and the sample was removed for processing. The edges of the sample were cut to facilitate ammonium persulfate diffusion. When copper was fully etched the underside was gently lifted off and scarified while the upper side was kept. As described for direct transfer of multilayer graphene [13], two syringes were used simultaneously: one to pump the etching solution and the other to inject deionized water. This procedure was repeated until the water bath became clean from residual etchant. Thereafter, the substrate was placed in the bottom of the container below the floating sample and the liquid solution was withdrawn with the syringe to land the sample in the substrate. The sample was then kept for drying overnight at ambient temperature in a clean environment.
3. Results and discussion
Because the growth of CNWs does not require a catalyst, the role that it may have later in the process by enhancing or controlling it was overlooked. Therefore, the main criterion to choose an adequate substrate is its ability to sustain high temperature. Besides this, copper is well known for its low affinity to carbon compared to other materials [14], and it is heavily exploited in the synthesis of monolayer graphene [15]. In addition, extending the process of growth to several graphene layers is possible by tuning the experimental conditions [16]. Notably, in this work copper is found to promote a controllable growth from graphene layers to CNW-LFs in three steps using two different solid precursors: camphor, a volatile compound that turns directly from solid to vapour, and tetraacetylethane, which contains four oxygen and ten carbon atoms. During the first step of growth only a few layers of graphene begin coating copper followed by the formation of non-graphitizing carbons in the second step and then the nucleation of CNW-LFs in the last step. The resulting CNW-LFs have a nearly hemispherical geometry (figures 1(f) and (g)), and are barely distinguishable at high density because of the overlapping fringes (figures 1(a) and (b)). Their edges point in a perpendicular direction to the supporting surface. The process needs a high purity of hydrogen which acts as both a diluent gas for the precursors and an etching agent to prevent fringes from crosslinking or fast coalescence as explained in the next sections.
Figure 1. SEM characterizations of CVD-grown CNW-LFs at different stages. (a), (b) High density of CNW-LFs grown on multilayer graphene and transferred onto SiO2; (b) a magnified area where the fringes overlap and stack. (c) Transferred sample on SiO2 showing the early stage of CNW-LFs growth. The areas numbered 1, 2 and 3 correspond to successive layers of turbostratic graphene while the dark areas represent non-graphitizing carbons; (d) at an advanced stage of growth, non-graphitizing carbons cover almost the whole scanned area within numerous CNW-LFs standing right up. (e) A magnified image that shows the flower shape of CNW-LFs. (f) The white arrows indicate fringes coming out from nanosized fullerenic particles. (g) Magnified image of the area within the dashed white rectangle in image (f) in which successive nucleation of CNW-LFs have approximately hemispherical geometry.
Download figure:
Standard image High-resolution imageThe CVD synthesis of graphene on polycrystalline copper is always accompanied by numerous small islands corresponding to the nucleation of subsequent layers. The occurrence of these nucleation sites is boosted by the high flux of active carbon fragments generated in the chamber and transformed into turbostratic stacking of graphene planes (figure 1(c)). However, unlike the first layer for which the growth rate is faster due to the dissociation of active carbon on the Cu surface [17], the growth rate of the next layers is decelerated and their numbers vary from one site to another. Indeed, the image in figure 1(c), corresponds to an incomplete growth and displays a variety of contrasts due to the non-uniform piling of graphene layers, particularly the dark zones which corresponds to non-graphitizing carbons. Even though the detailed structures of non-graphitizing carbons is still unknown to date, there is a general consensus that describes them as a network made up of microstructures that consist of carbon layer planes twisted and crosslinked through bridging groups and all fullerene-like in nature [18]. It is likely that the observed particles within different sizes and shapes in the dark areas (figures 1(f) and (g)) can also be considered fullerene-like structures. It is further possible to distinguish fringes of carbon coming out form the sides of these fullerenic particles (figures 1(f) and (g)) which is indicative of the nucleation phase of CNW-LFs. Some fringes form roughly hemispherical geometries and from their centres subsequent fringes rise and expand through the process (figures 1(e) and (g)). Other configurations consist of several petal-like graphite sheets surrounding the nucleation site within circular symmetry (supplementary figure S1). Both geometries have similar shapes to the flowers in figures 1(d) and (e) and suggest that the growth can be thought of in terms of both surface diffusion of carbon bearing species and expansion of fringes by reactive carbon arriving at the edges. Comparatively, this elucidates the proposed mechanism of CNW growth explaining that the curling force at the grain boundaries of nano-graphite domains, also called sheets or nanographene layers, causes the vertical growth [19–21]. More precisely, the curvatures are due to the presence of pentagonal carbon rings and other non-six membered rings into the prevailing honeycomb structure of carbon [22, 23]. Nevertheless, the transition phase of non-graphitizing carbons was not mentioned previously since it cannot be assessed without a TEM tool.
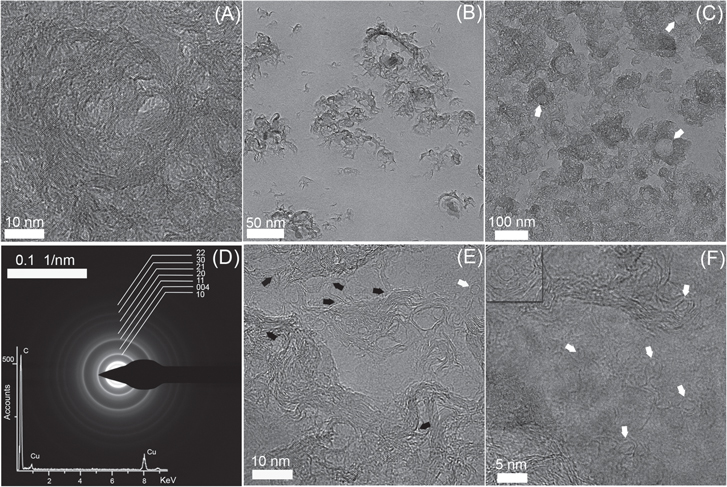
The hydrophobicity of the multilayer graphene and its small thickness enables a direct transfer of the synthesized graphenic material on a TEM grid for TEM characterizations without using any intermediate polymers, which can alter the cleanliness of samples. As shown in the TEM image in figure 2(a), it is quite difficult to distinguish between the fringes of CNW-LFs, non-graphitizing carbons, and fullerenic particles due to their high overlap. Only the upper fringes of CNW-LFs are visualized and consist of intertwined curved graphene layers, some of which are clearly organized in a more or less concentric arrangement around nucleus centres. The layers of fringes are highly disordered, curved, and contain a significant number of distortions with an average interlayer spacing of 3.36 Å (supplementary figure S2). The energy dispersive x-ray spectroscopy (EDS) analysis reveals that carbon is the only compositional element of the material (figure 2(d)). Moreover, measurements from selected area electron diffraction (SAED) were performed (figure 2(d)) and recorded from the region shown in figure 2(a). Even though there is more than one phase in the synthesized material, which may lead to superimposition of diffraction rings corresponding to a particular phase, the SAED pattern mainly consists of the (00l) crystalline reflections, and the two-dimensional (hk) reflections characteristic of turbostratic carbons [24, 25]. The main characteristic plane of the graphite (002) plane cannot be distinguished because it is overlapped by the zero order Laue zone.
Figure 2. TEM analysis of CNW-LFs at different stages of growth. (a) HRTEM image of CNW-LFs. (b), (c) TEM image corresponding to initial stage of non-graphitizing growth; (c) advanced stage of growth where small, medium and wide voids are indicated by white arrows. (d) SAED pattern obtained from (a). The material is oxygen-free as revealed by the elemental EDS analyses in the inset, collected in the same image. The copper peaks in the EDS spectrum are due to the scattering caused by the copper TEM grid supporting the material. (e) Crosslinking between shells and sheets supported by multilayer graphene. The black arrows indicate that one or more fringes are shared and the white arrow shows a fullerenic particle. (f) Nanosized clusters, shown by white arrows, with extended fringes on the graphene layer. The inset has dimensions of 9 nm × 9 nm and shows fullerenic nanoparticles.
Download figure:
Standard image High-resolution imageAt the early stage of CNW-LF growth, figure 2(b) shows that the process begins with a random distribution of crumbled, twisted and intertwined sheets of graphene around nucleus centres (supplementary figure S2). As for non-graphitizing carbons, once the number of these graphene sheets becomes greater, they tend to interconnect and be self-organized into circular voids (figure 2(c)). This may be understood considering the flexibility of graphene sheets to reduce surface energy at high temperature [26]. As the growth advances, the voids are filled with more graphene sheets and then CNW-LFs (supplementary figure S2). One would also expect to see nanoparticles, nanostructures or agglomerates according to the SEM images shown in figure 1, but in the TEM images only patterns aligned with the incident beam can be imaged. Therefore, unfavourable orientations of entire or parts of nanostructures make them featureless to the electron beam. For this reason, only a few of the particles are visible (figures 2(e) and (f)) and many fringes seem to have discontinuities or unsaturated ends (supplementary figure S2). Regardless of the misorientation of some patterns, the crosslinking among fringes is a common feature and characteristic of non-graphitizing materials. Several neighbouring nuclei tend to fuse together by sharing most of their fringes, while others share only one or a few of their outer fringes (figure 2(e)). It could be viewed that the neighbouring nuclei coalescence or adhere to one another to minimize the surface energy. This remark can also be extended to graphene sheets that are connected by several fringes. In addition, HRTEM imaging on areas of low density of non-graphitizing carbons make a considerable number of small closed clusters visible that have a nearly circular form with extended tails (figure 2(f)). They were detected over the two-dimensional hexagonal graphene layer with sizes comparable to those of fullerenic particles. Similarly to previous works, two possibilities can be considered to explain their existence. It can be assumed that the small graphene flakes become curved under high temperatures which creates new bonds leading to a zipping up of the flakes' edges [27, 28]; or it is the result of self-assembled atoms or blocks of atoms moving towards the formation of thermodynamically-favoured fragments of fullerenes [29]. Once the very small fragments are formed, they undergo a bottom-up process through which motion and coalescence of blocks transform unstable clusters into giant fullerenes under high temperature [30, 31] or into more stable fullerenes, such as the ones indicated in figure 2(e) and in the inset figure 2(f). Accordingly, the hemispherical geometry of CNW-LFs is not spontaneous and the fullerenic nature of the nanoparticles or the agglomerates promotes the incipient form of CNW-LFs. Nevertheless, more investigations are needed to sustain this hypothesis which is beyond the scope of this work.
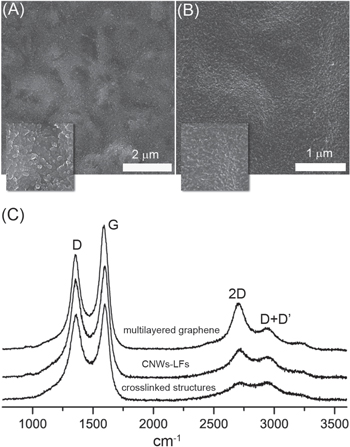
For carbon nanowalls, generally grown using hydrogenated feedstock, hydrogen is crucial for an effective growth of CNW-LFs [7]. In fact, a hydrogen pressure of 10 Torr was enough for tetraacetylethane to grow CNW-LFs while a pressure of 400 Torr was required for camphor to reproduce the same result. It is likely that the presence of oxygen in the chemical composition of tetraacetylethane contributes significantly to its decomposition, thus less hydrogen is needed. This is consistent with the conclusion that adding oxygen to a carbon source leads to an effective growth and high crystallinity of CNWs [20]. At low pressure of hydrogen, crosslinking and fast coalescence takes place and stops the process at the non-graphitizing step, so that other carbonaceous configurations are formed instead. The suppression of CNW-LFs occurs precisely when the edges of the fringes bond to each other, leading to seamless structures (figure 3(b)), or when the graphene sheets curl up and enclose into the shape of distorted particles (figure 3(a)). Clearly, high hydrogen pressure passivates the dangling bonds in the transition step of non-graphitizing carbons/CNW-LFs.
Figure 3. Crosslinked structures and Raman characterizations. (a), (b) the two kinds of crosslinking features encountered during the performed CVD growth at low hydrogen pressure are displayed. In (a) the fullerenic particles coalesce and stack randomly upon each other with noticeable gaps between them as shown in the inset, while in (b) the fullerenic particles crosslink smoothly to form seamless structures where only a few gaps are seen, as shown in the inset. (c) Raman spectra taken of transferred graphenic material on SiO2 corresponding to different types of structures: crosslinked structures, CNW-LFs and multilayer graphene. The multilayer spectrum of graphene was taken on a sample before the beginning of the non-graphitizing step. The measurements were carried out using a confocal Renishaw system with a 514 nm laser wavelength and 100× objective. The scale of the insets is 1 μm × 1 μm.
Download figure:
Standard image High-resolution imageRaman spectroscopy was performed on samples of CNW-LFs, multilayer graphene, and crosslinked structures resulting from low hydrogen growth. As shown in figure 3(c), the spectra are dominated by the carbon bands 2D, G and D which appear respectively near 2708, 1596 and 1355 cm−1. It is well known that these peaks are common features in spectra of sp2 bonded carbons [32]. The broadening and the increasing of D peak intensity from multilayer graphene to crosslinked structures plus the appearance of the D + D' peak around 2946 cm−1 are a consequence of the high disorder introduced by non-graphitizing carbons and CNW-LFs [32–34]. Moreover, the expected D' peak around 1620 cm−1, characteristic of disordered graphite structures, overlaps with the G peak. Any attempt to distinguish between the three different phases by curve fitting would not be accurate due to the complex morphology of non-graphitizing carbons. There is also a 2D' peak around 3250 cm−1 which is the second order of the D' peak. Its presence is usually expected in graphitic structures whether defects exist or not [33]. In addition, the second order peaks, 2D and 2D', broaden from multilayered graphene to CNW-LFs to crosslinked structures which represents a consistent decrease of crystalline domain sizes [35]. However, CNW-LFs exhibit considerable crystallinity compared to the crosslinked structures due to the high pressure of hydrogen.
4. Conclusions
In summary, this study shows that multilayer graphene behaves as a platform to grow CNW-LFs through non-graphitizing carbons and fullerenic particles. The process begins with the growth of turbostratic graphene layers on copper followed by a transition step characterized by non-graphitizing carbons where fullerenic nanostructures are highly disordered and randomly oriented and sized. Afterwards, CNW-LFs are formed from the fullerenic particles or structures assisted by high pressure of pure hydrogen to avoid crosslinking and fast coalescence. However, tetraacetylethane, a precursor involving oxygen atoms, requires less hydrogen pressure compared to camphor which is a hydrocarbon, but both lead to the same quality of CNW-LFs. In addition, the hydrophobicity of the multilayer graphene and its thin thickness allow the transfer of the graphenic material into any substrate including TEM grids, which offers the possibility to explore carefully and easily wide areas of the suspended material under HRTEM. Overall, the findings of this work can help to investigate the full potential of CNWs, and highlight the role of multilayer graphene as a platform to synthesize new kinds of graphene-based materials.
Acknowledgments
The authors acknowledge financial support from The Natural Sciences and Engineering Research Council of Canada and The Fonds de Recherche du Québec—Nature et Technologies.