Abstract
Using anodic aluminum oxide membranes as the nanoreactors and controller, oriented nanowire arrays of the diluted magnetic semiconductor Mn-doped CuO have been successfully fabricated using Mn(NO3)2 · 4H2O and Cu(NO3)2 · 3H2O as the starting materials. X-ray diffraction measurements showed that the as-prepared oriented nanowire arrays are of high purity. Scanning electron microscope and transmission electron microscope studies showed the nanowires are oriented, continuous and uniform with a diameter and length of about 170 nm and several tens of micrometers, respectively, and thus of a high aspect ratio. Low-temperature magnetic measurements showed the ferromagnetic property of the oriented Mn-doped CuO nanowire arrays with the critical temperature at around 80 K, which will endow them with great potential applications in spintronics in the future.
Export citation and abstract BibTeX RIS
Introduction
Diluted magnetic semiconductors (DMSs) [1], which combine semi-conducting with magnetic properties, are considered to be promising materials for their significant application in memory devices, detectors, light-emitting sources and the next generation of spintronic devices [2], which derive from the operation of not only the charge but also the spin of an electron [3]. As is well known, pure CuO is one of the most typical p-type metal-oxide semiconductors [4, 5] and exhibits interesting antiferromagnetic ordering below its Néel temperature of 225 K. It has been proved that the Mn ion is an excellent magnetic dopant [6–9]. Recently, doping the Mn ions into the monoclinic lattice to substitute for a fraction of the Cu ions has been shown to make CuO possess DMS properties [10, 11].
Since our discovery of the ferromagnetic characteristic of bulk Mn-doped CuO synthesized through a co-precipitation method followed by the annealing process [11], a few reports have been published on the fabrication of Mn-doped CuO with different morphologies in different ways. Hussain et al have synthesized Mn-doped CuO nanoparticles by the co-precipitation method and analyzed their ferromagnetism origin [12]. Sharma et al have prepared Mn-doped CuO amorphous nanoparticles by a hydrothermal method and then studied their weak ferroelectricity and ferromagnetism properties [13]. Mariammal et al have synthesized Mn-doped CuO nanoflakes by a wet chemical method and also discussed their ferromagnetism origin [14]. Zhu et al have fabricated highly (111) oriented Mn-doped CuO thin films on a thermally oxidized silicon substrate by radio-frequency magnetron sputtering [15]. Gülen et al have deposited Mn-doped CuO thin films on glass substrates via a successive ionic layer adsorption and reaction method and measured their optical band gaps [16]. Recently, Mn-doped CuO with the nanowire morphology has been successfully synthesized in our group through thermal oxidization of Mn–Cu alloys [17]. However, these nanowires were distributed randomly rather than oriented on the Cu2O pieces. And the following magnetic measurements showed strong Mn3O4 background magnetic signals. These two shortcomings may limit their potential applications in nanoscale spintronic devices. Therefore, research on the fabrication of Mn-doped CuO nanowires with well-defined orientation and high purity still remains a great challenge. As known to us, many methods have been developed to prepare one-dimensional nanostructures, such as solid state reaction [18], thermal evaporation [19] and the hydrothermal method [20]. However, the template method, especially based on a porous anodic aluminum oxide (AAO) membrane, is still a simple, facile and effective strategy to synthesize one-dimensional nanostructures [21, 22]. Furthermore, unlike other methods, the AAO membrane consists of numerous parallel and straight nanoscale channels, which not only provides the optimal conditions to synthesize one-dimensional nanostructures but also can automatically make the nanostructures arrange into arrays so that their physical properties sometimes can be significantly enhanced [23]. Using AAO membranes to prepare oriented one-dimensional nanostructures is still a good choice, especially for materials which are rather difficult to form into one-dimensional morphologies.
In the present work, in aid of the oriented nanochannels of the AAO membranes, Mn-doped CuO nanowire arrays with high purity have been successfully prepared using Mn(NO3)2 · 4H2O and Cu(NO3)2 · 3H2O as the starting materials. Oriented Mn-doped CuO nanowire arrays possess good ferromagnetic characteristics, which will endow them with great potential applications in the field of spintronics in the future.
Experiment
Mn-doped CuO nanowire arrays were prepared with the assistance of AAO membranes. All chemical reagents used in our experiment were of analytical grade and were used as received without further purification.
Preparation of AAO membranes
The AAO membranes were prepared by a fast two-step anodization method as reported in previous work [21]. Briefly, high-purity aluminum foil (purity 99.99%) was annealed at 600 °C for 5 h in open air to eliminate its inner stress and then degreased with acetone. After being corroded by a 0.1 mol l−1 NaOH solution for 30 s, the as-treated aluminum foil was firstly anodized in a 0.3 mol l−1 H2C2O4 aqueous solution at 0 °C under a constant DC voltage of 50 V for 10 min that was then uniformly increased to 120 V within 2 min before the foil was anodized for another 90 min. After anodization, the membranes were immersed into a 0.8 mol l−1 phosphoric acid solution at 30 °C for 40 min to widen their pores. Finally, the membranes were rinsed with distilled water many times and dried in an oven.
Synthesis of Mn-doped CuO nanowire arrays
The membrane with the remaining aluminum substrate was transferred into the bottom of a 50 ml porcelain crucible with the membrane facing upwards. From the results of bulk Mn-doped CuO [11], a 15 at.% Mn-doped sample has the biggest Ms value. Accordingly, the identical Mn concentration of 15 at.% was chosen for the Mn-doped CuO nanowire arrays. In this experiment, 3 mmol Mn(NO3)2 · 4H2O and 17 mmol Cu(NO3)2 · 3H2O were mixed and ground thoroughly in an agate mortar. Subsequently, the obtained blue mixture was put onto the AAO membrane in the porcelain crucible, which was later heated in a muffle furnace. Then the temperature was increased from room temperature to 400 °C at a slow rate of 5 °C/min and maintained for 10 h. After being cooled down to room temperature naturally, the AAO membrane was taken out carefully and the black material, covered on the surface, was cleaned completely. In order to fill optimally the pores of the membrane, this process was repeated three times. Finally, the black membrane was annealed at a higher temperature of 600 °C for another 10 h and cooled down to room temperature naturally. Meanwhile, in order to illustrate the reproducibility of the used method, a sample with 8 at.% Mn dopant was prepared as a reference.
Characterization
The morphologies of the AAO membranes were studied by a scanning electron microscope (SEM, Environment Quanta 200 with energy dispersive x-ray analysis-EDAX). The phase structure of the nanowire arrays was collected by an x-ray diffractmeter (XRD, TD-3500) with Cu Kα radiation (λ = 1.542 Å) at an operating voltage of 30 kV and current of 20 mA. The morphologies of the as-synthesized nanowire arrays were investigated by both SEM and a transmission electron microscope (TEM, JEM-1200EX), respectively. The magnetic properties were measured by a Physical Property Measurement System (PPMS, Model 6000) at low temperature.
Results and discussion
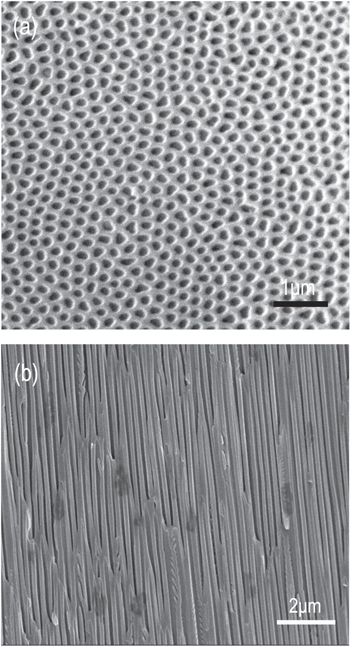
Figure 1 shows typical SEM images of the morphologies of the used AAO membranes after pore widening. As shown in figure 1(a), the pores are rather uniform and their average diameter after widening for 40 min at 30 °C in a 0.8 mol l−1 phosphoric acid solution is about 170 nm. The thickness of the AAO membranes is up to about 50 μm, which was also confirmed by SEM. Figure 1(b) shows a typical cross-section SEM image of the AAO membranes, from which it can be seen that almost all of the nanochannels are basically continuous, parallel and straight from top to bottom. However, it should be noted that parts of some nanochannels are covered by the wall of the nanopores so that they seem 'non-continuous' in the side-view SEM image. Moreover, during the electrochemical anodization process, it is a normal phenomenon that a small part of the AAO nanochannels displays bifurcate morphology, such as a 'Y' shape and a serrate structure [24]. Thus, the obtained AAO templates with their hollow, continuous, parallel and straight nanochannels can be exploited to fabricate oriented nanowire arrays.
Figure 1. Top-view (a) and side-view (b) SEM images of a blank AAO membrane after pore widening.
Download figure:
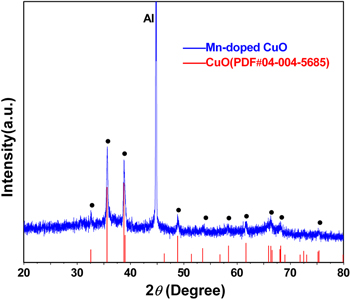
Standard image High-resolution imageThe crystal structure of the Mn-doped CuO nanowire arrays within the AAO membranes has been carefully investigated by using XRD and a typical XRD result is depicted in figure 2. From the figure, it was understood that the samples are well crystallized. The peak positions were found to be in accordance with the monoclinic structure of CuO [JCPDS card: 04-004-5685]. Meanwhile, it's evident from the XRD data that there are no additional peaks of manganese oxides, which means that the substitution of Cu ions in the CuO lattice by Mn ions has not changed its monoclinic structure. In addition, it's also evident that there are no extra peaks belonging to other copper oxides or any copper manganese oxides, indicating that the as-synthesized samples are of high purity. The antiferromagnetic material CuMn2O4 is a common accompanying phase when preparing bulk Mn-doped CuO samples [11, 12]. Its absence in the present samples may be attributed to the confinement of the AAO nanochannels. The AAO membrane is still in its amorphous state after being annealed at 600 °C [25] and the sharp and strong peak at 44.8° corresponds to the (200) plane of the remaining aluminum substrate [JCPDS card: 04-004-8743].
Figure 2. A typical XRD pattern of the Mn-doped CuO nanowire arrays within an AAO membrane.
Download figure:
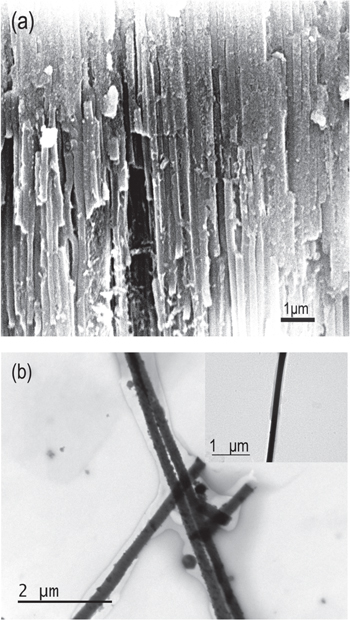
Standard image High-resolution imageAfter completely corrupting the remaining aluminum substrate in a mixed solution of CuCl2 and hydrochloric acid, the Mn-doped CuO nanowire arrays within the AAO membrane can be obtained for further cross-section SEM testing. Figure 3(a) shows a typical cross-section SEM image of the as-prepared sample, from which it can be clearly found that the hollow nanopores of the AAO membrane have disappeared compared with figure 1(b). Instead, they have been filled with concrete nanowires and most importantly, the nanowires are parallel with each other, forming oriented arrays. It can also be observed that some nanowires have been broken during preparation of the SEM sample so that their continuity was destroyed. The EDAX spectrum of the Mn-doped CuO nanowires within the AAO membrane has been collected by the SEM. The result shows that 14.96 at.% Cu has been substituted by Mn, which is highly consistent with our design. So this Mn-doped CuO sample can be written as Cu0.85Mn0.15O.
Figure 3. (a) Cross-section SEM image of Mn-doped CuO nanowire arrays and (b) TEM image of some Mn-doped CuO nanowires. The inset of figure 3(b) is a TEM image of a single nanowire.
Download figure:
Standard image High-resolution imageIn order to further investigate the morphology of the as-synthesized Mn-doped CuO nanowires, the AAO membrane was removed selectively by treating the sample with a hot NaOH aqueous solution. After being washed by distilled water several times, the Mn-doped CuO nanowires were dispersed into ethanol. Then, a drop of this ethanol was put onto copper grids coated with a thin carbon film for TEM study. Figure 3(b) shows typical TEM images of the Mn-doped CuO nanowires, from which it can be seen that the nanowires are rather smooth, uniform and compact. The inset of figure 3(b) is a TEM image of a single nanowire, from which it can be calculated that the diameter of the nanowires is about 170 nm, which closely corresponds to the pore size of the AAO membrane. Meanwhile, the nanowires are longitudinally continuous and their length is up to about several tens of micrometers so that they possess a high aspect ratio.
The chemical substances, Mn(NO3)2 · 4H2O and Cu(NO3)2 · 3H2O, are used as the providers of manganese and copper elements during the fabrication process. Both of them have relatively low melting points, which are 25.8 °C and 114.5 °C, respectively. Moreover, they can decompose to manganese and copper oxides at a certain temperature, which supplies us with a new way of filling the nanopores of the AAO membranes and further fabricating oriented nanowire arrays. The process can be briefly described as follows: (1) when the starting uniformly mixed materials Mn(NO3)2 · 4H2O and Cu(NO3)2 · 3H2O were heated to a temperature beyond their melting points, they could melt into fluid and then be injected into the nanopores of the AAO membranes through capillary action; (2) when the temperature increased to higher than their resolving points, the nitrates would decompose to their corresponding oxides in the nanopores; (3) as annealed at a higher temperature, the oxides of manganese and copper would react with each other and oriented Mn-doped CuO nanowire arrays could be formed with the confinement of the AAO nanochannels.
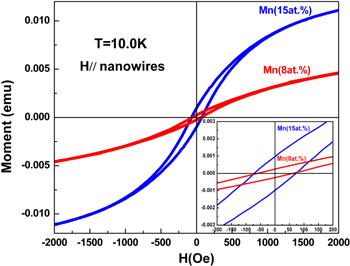
The magnetic properties of the Mn-doped CuO nanowire arrays were characterized by PPMS at low temperature. The M-H curves were measured with the applied magnetic field parallel and vertical to the nanowires, respectively. Figure 4 shows the M-H curves of the nanowire arrays at 10 K with the applied magnetic field parallel to the nanowires. The hysteresis loop, the unique characteristic of the ferromagnetic behavior, is clearly observed, from which it can be directly deduced that ferromagnetism was realized in the prepared samples. The M-H curve is almost the same when the applied magnetic field is perpendicular to the nanowires which means that the nanowire arrays formed in the AAO membrane exhibit the characteristic of magnetic isotropy, which is quite consistent with the results in [22]. This may come from the fact that the prepared Mn-doped CuO nanowires are made up of many fine nanoparticles. The inset of figure 4 shows us the central part of magnified hysteresis loops at 10 K, from which the coercivity (Hc) of the synthesized nanowire arrays can be measured to be about 70 Oe. The hysteresis loop shows no tendency to saturate even at a high magnetic field of 15 kOe, indicating the presence of the paramagnetic aluminum and AAO membrane along with the ferromagnetic phase. A similar result has also been obtained for the same area of the nanowire arrays embedded in the AAO membrane with the 8 at.% Mn dopant, which is also shown in figure 4. This reveals it is repeatable for the preparation of ferromagnetic Mn-doped CuO nanowire arrays. Very different from that of our previously obtained Mn-doped CuO nanowires synthesized by the thermal oxidation of Mn–Cu alloys [17], the shape of the hysteresis loop here is rather symmetric without any obvious distortion, which reveals the high magnetic purity of the prepared samples. As known to us all, pure CuO is an antiferromagnetic material with a Néel temperature of 225 K. The aluminum substrate and AAO membrane don't possess ferromagnetic characteristics. The only ferromagnetic material among the manganese oxides is Mn3O4, whose existence can be excluded by the XRD pattern and the highly symmetric shape of the hysteresis loop. The origin of the ferromagnetic signal at 10 K can be attributed to the Mn-doped CuO nanowire arrays despite the mechanism of ferromagnetism still not being very clear at present [14]. Gao et al proposed that the origin is due to the formation of bound magnetic polarons (BMPs) in bulk Mn-doped CuO semiconductors [26]. Rao et al assigned the ferromagnetic properties in Mn-doped CuO microstructures to the coexistence of Mn2+ and Mn3+ ions via the double exchange mechanism [27]. However, Zhao et al concluded that the super-exchange interaction of Mn–O–Cu–O–Mn coupling is responsible for the origin of ferromagnetic properties in Mn-doped CuO thin films [10]. Our obtained Mn-doped CuO nanowire arrays are made up of a lot of fine nanoparticles which is similar to that in [26]. Consequently, it is regarded that the origin of the ferromagnetism has the identical mechanism with that case. The ferromagnetism may arise from the formation of BMPs, which consist of a localized electron and many Mn2+ ions around the electron localization center. At temperatures below TC, the overlapping and interaction of neighboring BMPs produce long-range ferromagnetic coupling and therefore macroscopic ferromagnetism appears in our Mn-doped CuO semiconductor nanowires.
Figure 4. M–H curves measured at 10 K of the Mn-doped CuO nanowire arrays within the AAO membrane. The magnetic field was applied parallel to the nanowires. The bottom right inset shows the magnification of the central part of the M–H curves.
Download figure:
Standard image High-resolution imageIn order to further ascertain the magnetic phase and find out the critical temperature of the Mn-doped CuO nanowire arrays, the field-cooled (FC) and zero field-cooled (ZFC) magnetization curves were measured between 10 K and 180 K in an applied magnetic field of 100 Oe parallel to the nanowires. Figure 5 shows the ZFC and FC M-T curves of the prepared 15 at.% Mn-doped CuO nanowire arrays. From the M-T measurement results, it can be found that the FC and ZFC curves show divergence at around 80 K. The magnetization increases rapidly in the FC curve which reveals the existence of ferromagnetism in the Mn-doped CuO nanowire arrays. Meanwhile, only one critical temperature can be observed around 80 K, which can be assigned to the paramagnetic to ferromagnetic transition. The critical temperature TC is about 80 K, which is largely consistent with the previously reported results of Mn-doped CuO materials [11, 12]. As is known, the Curie temperature of the ferromagnetic material Mn3O4 is about 43 K and this result further excludes its existence in our sample. Therefore, it can be deduced that the magnetic phase in the sample is Mn-doped CuO with a magnetic critical temperature around 80 K. Below this temperature, the Mn-doped CuO nanowire arrays present ferromagnetic properties.
Figure 5. Temperature dependence of the magnetization of the 15 at.% Mn-doped CuO nanowire arrays within the AAO membrane under a magnetic field of 100 Oe applied parallel to the nanowires.
Download figure:
Standard image High-resolution imageConclusions
In summary, oriented Mn-doped CuO nanowire arrays were fabricated using Mn(NO3)2 · 4H2O and Cu(NO3)2 · 3H2O as the starting materials with the assistance of an AAO membrane. SEM and TEM studies confirm the morphology of oriented nanowire arrays with a high aspect ratio. Magnetic measurements at low temperature reveal their typical ferromagnetic properties with a critical temperature of around 80 K. The oriented DMS Mn-doped CuO nanowire arrays will find great potential applications in spintronics in the future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 61176087).