Abstract
Thermal energy storage based on adsorption and desorption of water on an adsorbent can achieve high energy storage densities. Many adsorbents lose adsorption capacity when operated under unfavourable hydrothermal conditions during adsorption and desorption. The stability of an adsorbent against stressing hydrothermal conditions is a key issue for its usability in adsorption thermal energy storage. We built an experimental setup that simultaneously controls the hydrothermal conditions of 16 samples arranged in a matrix of four temperatures and four water vapour pressures. This setup allows the testing of potential adsorbents between temperatures of 50 °C and 350 °C and water vapour pressures of up to 32 kPa. A measurement procedure that allows the detection of the hydrothermal stability of an adsorbent after defined time spans has been designed. We verified the functionality of the multiple sample measurements with a microporous adsorbent, a zeolite NaMSX. The hydrothermal stability of this zeolite is tested by water uptake measurements. A standard deviation lower than 1% of the 16 samples for detecting the hydrothermal stability enables setting different conditions in each sample cell. Further, we compared the water uptake measurements by measuring their adsorption isotherms with the volumetric device BELSORP Aqua 3 from Bel Japan.
Export citation and abstract BibTeX RIS
1. Introduction
Thermal energy storage (TES) devices can balance the supply and demand of energy and are able to increase the efficiency of an energy system. The reversible process of adsorption can be used for storing thermal energy [1]. Solid adsorbents can adsorb gaseous materials, so-called adsorptives, whereby the heat of adsorption is released. The adsorptive is bound to the adsorbent in a condensed phase called the adsorbate [2]. When heat is supplied to the adsorbent, the adsorbate is desorbed from the adsorbent and released as a gaseous phase.
Adsorption systems can be used as thermal heat pumps [3–5] or for thermal energy storage [6–8]. Hydrophilic adsorbents such as zeolite 13X or 4A proved to be suitable adsorbent materials for TES [9]. High water uptake and high temperature lifts (e.g. ΔT > 100 K) of the carrier gas during the adsorption process were measured [9, 10].
In addition to the sorption capacity, with regards to lifetime, the hydrothermal stability is a key issue for a suitable adsorbent. Degradation of some adsorbents because of high temperatures in the presence of water vapour has been known since the 1960s [11]. The chemical mechanism underlying the decomposition is not completely understood yet. Lutz et al describe different chemical mechanisms depending on the aluminium content of zeolites [12].
A few publications regarding testing the hydrothermal stability of adsorbents for use in heat storage or transformation processes are available [13–19]. Different adsorbents in different forms (powder, beads and coats) were examined by repeating the adsorption and desorption up to several thousand cycles. In general, the procedure for testing the hydrothermal stability consists of two parts. First, the sample is hydrothermally stressed. Afterwards, the potential degradation of the sample is investigated. The detection is commonly done by comparing the water sorption capacity before and after the hydrothermal stress. A reduction in the capacity correlates with a decrease in the energy storage density.
Currently, no apparatus is commercially available for testing the hydrothermal stability. The Henninger research group analyses the hydrothermal stability of adsorbents for adsorption heat pumps and chillers [15]. A combination of a thermogravimetric apparatus (Setaram TG/DSC 111) and a humidity generator (Setaram Wetsys) is used for performing short-term stability tests (up to 30 cycles) [20]. In this setup, the sorption capacity can be measured in situ. However, only one sample can be characterized at once. For testing the long-term cycle stability (up to 50 000 cycles) an apparatus with three sample chambers for fast cycling was built [15]. The sample chambers are designed to hold adsorbent coatings. For the characterization of the hydrothermal stability, a magnetic suspension balance from Rubotherm is used. Freni et al report an experimental setup for the simultaneous cycling of 15 samples in an open system for heat pumping applications [18]. The stability is checked by measuring the water adsorption isobars of the samples with a thermogravimetric microbalance. Storch et al describe an instrument for cycling 8 samples in an open system [14]. The samples are inspected for their usability in heat storage. Thus, higher temperatures (up to 350 °C) can be set in this apparatus. The state of the samples is, among others, determined by measuring the water uptake in a desiccator.
1.1. Motivation
The hydrothermal degradation of adsorbents depends on the temperature, the water vapour pressure and the duration of the exposure (see e.g. [12]). For testing the hydrothermal stability of a potential adsorbent, the discharging and charging modes of the application have to be experimentally emulated. The result is not readily transferable to other adsorption and desorption conditions, e.g. higher water vapour pressure during desorption. According to the application, up to several thousand cycles are necessary for characterizing a possible candidate as an adsorption material. Thus, testing the hydrothermal stability of adsorbents is still very time-consuming.
In order to reduce the time for detecting the right adsorbent for a defined application we propose the following method. The hydrothermal stability of an adsorbent is tested within a broad temperature and water vapour region and for defined times. Then, the obtained experimental data is used for setting up a model equation. This model can be applied for the prediction of the hydrothermal stability of the absorbent in different applications. As a consequence no further experimental time will be required.
1.2. Objective
For this proposed method, comprehensive and precise experimental data of the hydrothermal stability is necessary. For gathering the required data, a multiple sample setup was built, which shall allow studying the influences of the temperature, the water vapour pressure and the duration on the hydrothermal stability of an adsorbent. In contrast to the cycling tests mentioned above, the temperature and the water vapour pressure on the samples are maintained constant during the hydrothermal stress. After defined time spans, the degradation is measured by water uptake measurements. The constant conditions of temperature and pressure offer the possibility of modelling the stability.
The purpose of this paper is to present the functionality and the accuracy of the multiple sample setup. We first present the experimental setup and the measurement procedures. Second, we identify the reproducibility and measurement uncertainties of the multiple sample measurements. Third, the water uptake measurements of the experimental setup are compared with the adsorption isotherms determined using a volumetric measurement device.
2. Experiment
2.1. Experimental setup
The experimental setup allows varying the temperature, water vapour pressure and duration of the exposure independently. A sample material of an adsorbent is placed in 16 different cells. Groups of four cells are placed in one of four heated, nearly isothermal aluminium blocks with temperatures T1–T4. One sample cell of each isothermal block is connected to one of four temperate water reservoirs with the water vapour pressures P1–P4. Thus, 16 different data points can be recorded at once (figure 1).
Figure 1. Four water vapour pressures and four temperatures allow the recording of 16 different conditions.
Download figure:
Standard image High-resolution imageAfter a defined time, the water uptake of each sample is externally measured gravimetrically. The decrease in water uptake corresponds to the hydrothermal degradation of each sample. Figure 2 shows a sketch of the experimental setup with one of four temperate water reservoirs. A photograph of the experimental setup is given in figure 3.
Figure 2. Sketch of the experimental setup (One of four temperate water reservoirs is shown).
Download figure:
Standard image High-resolution imageFigure 3. Photograph of the experimental setup with the insulated blocks (T1–T4), the water reservoirs (P1–P4), quick connections to the sample cells (1) and pneumatic valves (2).
Download figure:
Standard image High-resolution imageThe temperature range of each block is between 30 °C and 350 °C ± 0.7% of the reading. This measurement uncertainty results from the uncertainties of the temperature sensor calibration, the data acquisition system and potential temperature variations across an aluminium block. Influences on the temperature of one block to another could not be observed. The temperature can be set by three heating cartridges per block and is measured using two PT-100 RTD sensors. One sensor is below the sample cells, close to the bottom of the block and is also used for temperature control. The other sensor is close to the top of the block. The temperature difference of these sensors is less than 0.2% of the reading. The blocks are insulated with 25 mm-thick high-temperature thermal insulation.
Each sample cell is connected to one of four temperate water reservoirs via an automatic pneumatic valve. The water reservoirs stand in double-walled aluminium bins, which are insulated with 38 mm-thick thermal insulation to ensure a homogeneous temperature distribution. The temperature of each water reservoir can be set between 5 and 70 °C ± 0.1 K using a peltier element. The temperature of the water reservoirs is measured using a PT-100 sensor that is located in the centre of the water reservoir. The temperature difference between the chamber and the bottom of the reservoir, where the peltier element is placed, leads to a small vertical temperature gradient inside of the water reservoir, which is less than 0.1 K.
The water vapour pressure of each water reservoir is measured using a capacitance manometer model 730 from Setra Systems. The pressure range is 100 kPa with an accuracy of ± 0.25% of the reading. The pressure in the system is below the atmospheric pressure. Thus atmospheric gas can enter the system in case of leakages and prevent the water vapour transport from or to the samples. By comparing the temperature of the water reservoir and the water vapour pressure, it is possible to detect a pressure increase due to a leakage in the system.
The temperate blocks, the water reservoirs and the manometers are situated in a heated chamber with a temperature higher than that of the water reservoirs to prevent condensation. The system (samples or water reservoir) is evacuated with a rotary vane pump, which reaches a final pressure of 2 × 10 − 4 kPa. The evacuation of the samples can be done either via a needle valve or a stop valve. The needle valve is used at the beginning of the evacuation at pressures close to the atmosphere to prevent fluidization of the sample. At pressures below 0.1 kPa the stop valve is also opened to decrease evacuation time. Additionally, a metal sinter filter with a pore width of 2 µm is installed above the sample cell (see figure 4).
Figure 4. Photograph of one sample cell with filter, manual membrane valve and quick connect.
Download figure:
Standard image High-resolution imageBefore a sample cell is disconnected using a quick connect from the experimental setup for weighing, the manual membrane valve is closed. This valve is installed above the sinter filter and below the quick connect. It prevents atmospheric gas from flowing into the cell, while the sample cell is disconnected. The water uptake of the samples is detected externally using a precision balance with a reproducibility of ⩽ ±0.7 mg. The weight of the total arrangement of sample cell, filter, manual membrane valve and quick connect is about 550 g.
2.2. Measurement procedure
Each of the 16 sample cells are filled with about 5 g of adsorbent beads. The measurement procedure for testing the hydrothermal stability is illustrated in figure 5. By degassing the samples in the cells we obtain the dry mass md. During this time, the valves V2 and V3 are opened and V1 is closed (see figure 2). The degassing is performed in three steps to avoid the unintended ageing of the samples. At the beginning, the samples are degassed using the rotary vane pump until the pressure is less than 0.5 kPa. Then, an automated heating procedure is additionally used to desorb the final amount of water from the sample. For that purpose, the temperature is set to 150 °C with a holding time of 2 h, then 250 °C for 2 h and finally 350 °C for another 15 h. After cooling the sample cells to room temperature, they are disconnected from the system and their dry mass is measured using the precision balance.
Figure 5. Measurement procedure for testing the hydrothermal stability of an adsorbent.
Download figure:
Standard image High-resolution imageThe next step is to prepare the water reservoirs for the water uptake measurements. De-ionised water in a flask is put in an ultrasonic bath for 30 min to degas the water before it is filled in the reservoirs. Then, each water reservoir is evacuated with the vacuum pump three times for 30 s (V1 and V3 is opened, V2 is closed). Thereby the amount of dissolved gas is further decreased and the space above the water is evacuated. As soon as the leakage rates of the empty water reservoir and the water reservoir with the degassed water are very close, the water is adequately degassed and no further degassing is required.
The water uptake of the samples Xs what is the quotient of the mass of the adsorbed water mw and the mass of the dry adsorbent md is detected:

The temperatures of the four aluminium blocks with the sample cells are set at T = 50 °C and the four water reservoir temperatures are set at Tw = 36.7 °C corresponding to a relative water vapour pressure at the samples of:

One reason for choosing a relative water vapour pressure of 50% is that a change in the relative water vapour pressure has less influence on the water uptake than in regions of lower or higher relative water vapour pressures. Thus, the comparison of the water uptake of the 16 samples is more precise. The typical shape of an isotherm of a microporous adsorbent such as zeolite has a steep increase at low and high relative water vapour pressures and small changes at medium relative water vapour pressures (see e.g. figure 9). A further reason is that these conditions allow a comparison to the water vapour adsorption isotherms measured with the BELSORP apparatus.
The valves V1 and V2 are opened, the valve V3 is closed until the samples reach the adsorption equilibrium. This equilibrium state cannot be detected using the experimental setup. It is checked by increasing the time the samples could adsorb water and comparing afterwards the adsorbed mass weighted with the balance. As soon as no change in the adsorbed mass of the samples is detected, the equilibrium is reached. The difference between the mass of the samples after the water uptake measurement and the mass of the dry samples md is equal to the mass of the adsorbed water mw of each sample.
Following this, the sample cells are placed in the aluminium blocks again. Then the degassing of the samples is performed again to prevent hydrothermal stress of the samples during the heating of the cells up to the desired temperature (see figure 5). The temperature of each block and each water reservoir is set to the conditions of the hydrothermal stress. As soon as the conditions are constant, the valves between the sample cells and the water reservoirs (V1 and V2) are opened for a defined time span, causing degradation according to the condition in each cell.
After this time span, the reduction of Xs is investigated by the water uptake measurement with the same conditions as before (50% relative water vapour pressure at 50 °C). The hydrothermal stability of the adsorbent Z corresponds to the ratio of remaining Xs,2 to the original water uptake Xs,1 (see figure 5).
2.3. Measurement conditions of the startup procedure
For verifying the functionality of the experimental setup and the usability of the multiple sample measurements, a procedure with the same conditions for all 16 sample cells was performed. As a sample material zeolite NaMSX (sodium medium silicon zeolite X) from Chemiewerke Bad Köstritz GmbH (Germany) with a bead size over the range 2.5–5 mm was used. The zeolite beads are produced with about 18% of clay binder [21]. Corresponding to the measurement procedure shown in figure 5 the hydrothermal stability was tested at a temperature of 250 °C and a water vapour pressure of 19.93 kPa (equal to a water bath temperature of 60 °C). The duration of the exposure to the water vapour pressure and the temperature at the samples was set at 67 h. During that time, the water reservoirs were evacuated for a few seconds every 6 h. This evacuation prevents an increase in the atmospheric gas in the system. After 67 h the water uptake of the hydrothermally stressed samples (hereinafter called aged samples) was measured and compared to the new samples.
2.4. Validation of the experimental results
The water uptake of the new and aged samples measured using the experimental setup are compared by measuring the adsorption isotherms of the new and aged samples using the volumetric measurement device BELSORP Aqua 3 (BELSORP) from Bel Japan.
2.4.1. Sample preparation for the volumetric measurement.
About 400 mg of new and aged adsorbent beads are degassed using the device Belprep-flow from Bel Japan in combination with a rotary vane pump. The samples are evacuated until the pressure is at a minimum value of 0.01 kPa. In addition, an automated heating procedure is used to desorb the final amount of water from the samples. The temperature is set at 150 °C for 2 h, then 250 °C for 2 h and finally 350 °C for a further 15 h. After cooling the samples to room temperature their dry mass is measured using an analytical balance.
2.4.2. Measurement settings for the volumetric measurement.
The adsorption isotherm is measured at a temperature of 50 °C. In the first run, the equilibrium time at each measuring point is set to 500 s. In the second run it is set to 1000 s. The adsorption isotherm is automatically measured by the supplied software from BEL Japan.
3. Results and discussion
3.1. Detecting the adsorption equilibrium time
The adsorption equilibrium cannot directly be read during the water uptake measurement in the experimental setup. Thus, the time for reaching the equilibrium is determined in a kinetic experiment in advance. For defined time spans the samples are exposed to a temperature of 50 °C and a relative water vapour pressure of 50%. After each time span the water uptake of the samples is measured externally using the balance and compared to the adsorption isotherms measured by the BELSORP. After each weight measurement the experimental setup is evacuated and set to the same conditions of temperature and pressure, before the next time span starts.
Figure 6 presents the mean water uptake of the 16 sample cells filled with a new sample of zeolite NaMSX. Additionally, the water uptake determined from the adsorption isotherms measured by the BELSORP are illustrated. After 5.5 h, the mean water uptake measured using the experimental setup is equal to 0.263 kg kg − 1 and close to the values that are measured by the BELSORP. Further measurements after a total time of 24 h and 25 h show that the water uptake increases slightly.
Figure 6. Water uptake of new NaMSX measured after 1, 2, 3, 4, 5.5, 24 and 25 h preset equilibrium time using the experimental setup (filled diamonds) in comparison to the water uptake determined from the adsorption isotherms measured by the BELSORP with a preset equilibrium time at each point of the adsorption isotherm of 500 s (dashed line) and 1000 s (line). For each adsorption isotherm 23 points are measured until a relative water vapour pressure of 50% is reached. This results in a cumulative equilibrium time of about 3 h 12 min or 6 h 24 min.
Download figure:
Standard image High-resolution imageIn order to ensure that the adsorption equilibrium is attained, an increasing exponential decay function [22] is set up to estimate the final water uptake Xs,equil after infinite time:
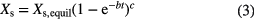
The measured values of the water uptake Xs are used as the independent variables in the non-linear regression analysis. The free parameters that have to be fitted in equation (3) are Xs,equil, b and c. The results are listed in table 1. The difference between the measured water uptake after 24 h and the calculated water uptake Xs,equil is minimal and within the error of the fit. The accuracy of the fit is good (R2 = 0.99). Consequently, we can set the time for reaching the adsorption equilibrium to 24 h.
Table 1. Measured water uptake, free parameters of the exponential decay function with errors and the coefficient of determination R2 of the fit.
| Adsorbent | Xs for 24 h (kg kg −1) | Xs,equil (kg kg −1) | Error of Xs,equil (kg kg−1) | b (h −1) | Error of b (h−1) | c(−) | Error of c(−) | R2(−) |
|---|---|---|---|---|---|---|---|---|
| NaMSX new | 0.2750 | 0.2783 | 5.5e-3 | 0.55 | 0.06 | 1.99 | 0.31 | 0.9942 |
| NaMSX aged | 0.2060 | 0.2069 | 2.9e-3 | 0.95 | 0.10 | 2.26 | 0.38 | 0.9960 |
Because of structural changes of the zeolite crystals after the hydrothermal stress, the adsorption equilibrium time may differ for new and aged zeolite. While on one hand the diffusion resistance of the aged zeolite should increase compared to the new zeolite [23], on the other, the water uptake of the aged zeolite is lower, which will decrease the integral heat of adsorption and possibly decrease the time for reaching the adsorption equilibrium.
Figure 7 illustrates the kinetic measurement of the aged zeolite NaMSX of the startup procedure (section 2.3). After 4 h, the mean water uptake, which was measured using the experimental setup, is close to the values measured by the BELSORP. However, a comparison of the water uptake measurements after 5.5 and 24 h shows that equilibrium was not attained. After 24 h the water uptake of the aged zeolite is equal to 0.206 kg kg − 1 and about 0.5% above the value of the BELSORP with an equilibrium time of 1000 s. The final water uptake Xs,equil is calculated using equation (3) for the aged NaMSX, analogous to the new NaMSX. The results are presented in table 1 and show that no quantifiable increase in the water uptake is expected with increasing times. Thus, the time for attaining the equilibrium can be set to 24 h for the aged NaMSX also.
Figure 7. Water uptake of aged NaMSX measured after 1, 2, 3, 4, 5.5, 24 and 25 h preset equilibrium time using the experimental setup (filled diamonds) in comparison to the water uptake determined from the adsorption isotherms measured by the BELSORP with a preset equilibrium time at each point of the adsorption isotherm of 500 s (dashed line) and 1000 s (line). For each adsorption isotherm 18 points are measured until a relative water vapour pressure of 50% is reached. This results in a cumulative equilibrium time of 2 h 30 min or 5 h.
Download figure:
Standard image High-resolution image3.2. Reproducibility and measurement uncertainties
The experimental setup is designed for setting different and accurate conditions (water vapour pressures and temperatures) in each of the 16 sample cells. This requires an excellent reproducibility and small measurement uncertainties. The reproducibility of detecting the dry mass and the water uptake of zeolite NaMSX in each sample cell was verified by repeating the degassing procedure as well as the water uptake measurement.
The maximum relative deviation of the dry sample mass between two measurements for all 16 samples is less than 0.05%. The maximum relative deviation of the adsorbed amount of water between two measurements for all 16 samples is less than 0.39%. The maximum expected measurement uncertainty of the water uptake ΔXs/Xs is defined as the sum of the measurement uncertainties of the mass of adsorbed water Δmw/mw and of the mass of dry adsorbent Δmd/md:
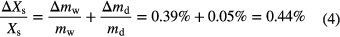
In literature, the uncertainty of detecting the water uptake using a Rubotherm thermobalance is reported to be 0.53% [24]. This value is calculated using a typical sample mass of 60 mg and a water uptake of 0.2 kg kg − 1. The uncertainty of measuring the water uptake using a combination of a thermogravimetric apparatus and a humidity generator from Setaram is reported as 0.43%, assuming a sample mass of 80 mg and a water uptake of 0.2 kg kg − 1 [24]. Thus, the measurement uncertainty of the multiple sample setup is comparable to these measurement uncertainties. However, the typical amount of sample that is needed for the characterization differs. The amount of sample that is placed in each of the 16 sample cells is about 5 g.
The hydrothermal stability Z is defined as the ratio of water uptake after the hydrothermal stress Xs,2 and water uptake before the hydrothermal stress Xs,1:

Thus, the measurement uncertainty of the hydrothermal stability for each sample cell is equal to:
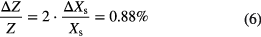
Differences in the water uptake measurement between the sample cells will be discussed in the following section.
3.3. Measurement of the water uptake of zeolite NaMSX
3.3.1. Results of the experimental setup.
Before the hydrothermal stability measurement was run, the water uptake of new zeolite NaMSX was measured at the reference water uptake conditions (50% relative water vapour pressure at a sample cell temperature of 50 °C) and an equilibrium time of 24 h. The measurement results of the 16 sample cells are plotted in figure 8. The error bars are calculated using the measurement uncertainty of equation (4). The standard deviation of all 16 samples is equal to 0.0018 kg kg − 1 or 0.7%, relative to the mean water uptake of 0.275 kg kg − 1. The standard deviation is on the same order of magnitude as the error bars. This confirms the hypothesis that all samples have the same water uptake under equal conditions.
Figure 8. Water uptake of new NaMSX measured using the experimental setup with an equilibrium time of 24 h.
Download figure:
Standard image High-resolution image3.3.2. Validation of the water uptake measurement.
Three adsorption isotherms of the new adsorbent NaMSX at 50 °C were measured using the BELSORP, see figure 9. The equilibrium time for each measured value of the adsorption isotherms was set to 1000 s. At a relative water vapour pressure of 50%, the mean water uptake is equal to 0.267 kg kg − 1 and the standard deviation is equal to 5.4 × 10 − 4 kg kg − 1. The water uptake measured using the BELSORP at the relative water vapour pressure of 50% is 3% below the mean water uptake measured using the experimental setup.
Figure 9. Adsorption isotherms at 50 °C of three samples of new NaMSX measured using the BELSORP with an equilibrium time of 1000 s for each measured value.
Download figure:
Standard image High-resolution imageThe difference in the water uptake can be explained by the different time spans that are set for reaching the thermodynamic adsorption equilibrium. The time until the water uptake was measured was set to 24 h using the experimental setup. The maximum time for reaching the equilibrium that can be set for each value of the adsorption isotherm using the BELSORP, is limited. The water uptake is calculated by measuring the pressure difference before and after a change of the relative water vapour pressure. As soon as the leakage rate of the system is of the same order of magnitude as the pressure difference, which would consequently falsify the result, the time for attaining the equilibrium cannot be increased.
3.4. Measurement of the hydrothermal stability of zeolite NaMSX
3.4.1. Results of the experimental setup.
After the 16 samples were hydrothermally stressed with the conditions as mentioned in section 2.3, their stability is detected using the reference water uptake measurement. The results are plotted in figure 10. The mean value of the water uptake is equal to 0.206 kg kg − 1 and the standard deviation is equal to 0.0017 kg kg − 1 or 0.8% of the mean water uptake.
Figure 10. Water uptake of aged NaMSX measured using the experimental setup with an equilibrium time of 24 h.
Download figure:
Standard image High-resolution imageThe measured dry mass of the aged adsorbent is slightly above the dry mass of the new adsorbent. That is still the case after increasing the degassing time up to 33 h at 350 °C. The mean value of the increased mass for the 16 sample cells is equal to 0.4% relative to the new adsorbent, with a standard deviation of 0.046%. Because of the hydrothermal stress, the crystalline structure of the zeolite is partly decomposed. Some of the water molecules are possibly trapped because of blocked exit pores and the desorption of these molecules is prohibited. Further adsorption and desorption measurements show that part of the irreversibly adsorbed amount of water can be desorbed after adsorption took place. Nevertheless, the dry mass of the aged adsorbent is still above the dry mass of the new adsorbent.
The result of the hydrothermal stability is illustrated in figure 11. The hydrothermal stability is equal to a mean value of 0.749 with a standard deviation of 0.0047. Consequently, the hydrothermal stability of an adsorbent in each sample cell can be detected with an uncertainty of better than 2% (3 times the relative standard deviation of 0.6%). It is expected that this uncertainty is the same under different ageing conditions in each cell. Therefore, redundant measurements do not seem to be necessary and 16 different conditions can be measured simultaneously.
Figure 11. Hydrothermal stability of the adsorbent NaMSX after 67 h of hydrothermal stress.
Download figure:
Standard image High-resolution image3.4.2. Validation of the hydrothermal stability measurement.
The adsorption isotherms of the aged samples of three sample cells 2, 3 and 13 were measured using the BELSORP. Figure 12 illustrates the results of these measurements. At a relative water vapour pressure of 50% the mean water uptake is equal to 0.205 kg kg − 1. The standard deviation is equal to 2.4 × 10 − 3 kg kg − 1.
Figure 12. Adsorption isotherms at 50 °C of three samples of the aged NaMSX measured using the BELSORP and an equilibrium time of 1000 s for each measured value.
Download figure:
Standard image High-resolution imageThe relative deviation between the water uptake measured using the experimental setup and the water uptake measured using the BELSORP is better than 0.5%.
3.5. Comparison of the adsorption isotherms of new and aged NaMSX
The hydrothermal stability is tested by measuring the reduction of the water uptake at a relative water vapour pressure of 50%. This procedure implies that the water uptake is reduced by the same factor along the relative water vapour pressure. This assumption was verified by Storch [25] and can again be substantiated by comparing the relative reduction of the water uptake of the new and aged adsorbent.
Therefore, the data points of the adsorption isotherms of the new and aged NaMSX are interpolated and compared at 20 different points within the relative water vapour pressure of 0.05 and 0.9. Figure 13 shows the adsorption isotherms of sample 3 and the relative reduction of the water uptake. The arithmetic average of the relative reduction is equal to 0.24 with a standard deviation of 0.005.
Figure 13. Adsorption isotherms of new (filled circle) and aged (open circle) NaMSX of sample 3 and the relative reduction of the water uptake of the new sample (open square).
Download figure:
Standard image High-resolution image4. Conclusions
A multiple sample setup for testing the hydrothermal stability of adsorbents has been developed. A measurement procedure has been designed that allows the detection of the hydrothermal stability of an adsorbent for defined hydrothermal conditions and after defined time spans. The functionality of the experimental setup was verified by comparing the water uptake measurements of the new and aged zeolite NaMSX samples with adsorption isotherms measured using the volumetric measurement device Belsorp Aqua 3. Repeated measurements with identical settings provide evidence of the reproducibility of the experimental setup.
The hydrothermal stability was determined with a standard deviation of 0.6% for a total number of 16 sample cells. This precision enables setting different conditions in each sample cell. Further, we verified that the water uptake of the zeolite NaMSX is reduced by the same factor along the relative water vapour pressure as a result of hydrothermal stress. Testing the hydrothermal stability of a zeolite with different conditions in each sample cell is currently in progress.
Acknowledgments
Financial support for this project (Grant No. 0327383B) by the German Federal Ministry of Economics and Technology is gratefully acknowledged.















