Abstract
The synthesis of gallium arsenide (GaAs) nanoparticles has attracted wide scientific and technological interest due to the possibility of tuning the GaAs NP (nanoparticle) band gap across the visible spectrum and their consequent use in optoelectronic devices. In recent years, laser ablation in liquid (LAL) has been widely used for the preparation of colloidal solutions of semiconducting and metallic nanoparticles, thanks to its flexibility. With the aim of highlighting the key role played by laser pulse duration on the ablation mechanism and on the properties of the obtained materials, laser ablation of a gallium arsenide target in acetone was performed using laser sources operating in two different temporal regimes: Nd:glass laser ( = 527 nm, pulse duration of 250 fs and frequency repetition rate of 10 Hz) and Nd:YAG laser (
= 527 nm, pulse duration of 250 fs and frequency repetition rate of 10 Hz) and Nd:YAG laser ( = 532 nm, pulse duration of 7 ns and frequency repetition rate of 10 Hz). The ablation process was studied following the dynamics of the laser induced shock waves (SWs) and cavitation bubbles (CBs) by fast shadowgraphy, showing that CB dimension and lifetime is related to the laser pulse length. A characterization of the obtained materials by TEM (transmission electron microscopy) and microRaman spectroscopy have shown that quite spherical gallium oxide/GaAs nanoparticles can be obtained by nanosecond laser ablation. On the other hand, pure polycrystalline GaAs nanoparticles can be produced by using an ultrashort laser source.
= 532 nm, pulse duration of 7 ns and frequency repetition rate of 10 Hz). The ablation process was studied following the dynamics of the laser induced shock waves (SWs) and cavitation bubbles (CBs) by fast shadowgraphy, showing that CB dimension and lifetime is related to the laser pulse length. A characterization of the obtained materials by TEM (transmission electron microscopy) and microRaman spectroscopy have shown that quite spherical gallium oxide/GaAs nanoparticles can be obtained by nanosecond laser ablation. On the other hand, pure polycrystalline GaAs nanoparticles can be produced by using an ultrashort laser source.
Export citation and abstract BibTeX RIS
1. Introduction
Gallium arsenide is a semiconductor widely used in optoelectronic devices and solar cells. It has a direct band gap of 1.42 eV at 300 K and a large Bohr exciton diameter of about 19 nm [1]. Nanoparticles (NPs) with a diameter smaller than the Bohr exciton one are usually termed quantum dots and present physical and chemical properties very different with respect to the bulk material. In this respect the synthesis of GaAs NPs has attracted scientific and technological interest due to the possibility of tuning the band gap across the visible spectrum and to observe luminescence at shorter wavelengths with respect to bulk GaAs. GaAs nanocrystals in the form of thin films or dispersed in oxide and polymeric matrices have been obtained both by chemical and physical methods. The described chemical synthesis of GaAs nanocrystals [2, 3] allows one to obtain a good size control and a narrow particle size distribution, but NPs tend to be prolate and with gallium in excess. Moreover, an amorphous oxide layer has been observed, due to the formation of Ga2O3 or As2O3 [3]. Among the physical methods, laser ablation either in vacuum or in the presence of buffer gases has been successfully used to obtain nanostructured GaAs thin films, using both nanosecond and femtosecond laser sources [4–7].
In recent decades the laser ablation in liquid (LAL) methodology has created widespread interest since it presents many advantages compared to other techniques. LAL has been widely used to obtain stable colloidal solutions of metals, alloys, ceramics, and semiconductors [8–10] in a quite simple route, without the use of harmful chemicals or stabilizing agents. Compared to laser ablation performed in vacuum or a gas atmosphere, in LAL experiments the expansion of the species of the laser-induced plasma generated at the solid–liquid interface is more strongly confined at the laser beam focal point. So, the local thermodynamic conditions can be expected to achieve more extreme values such as higher temperature and higher pressure than those obtained in vacuum or gases. Moreover, a higher cooling rate of the ablated material, due to the interaction with the surrounding liquid, can be expected. These extreme conditions can lead the laser-generated active species to react with either each other or with the molecules of the liquid medium.
The ablation in the liquid process has been described considering that, soon after the laser target interaction, the formation of a laser-induced plasma takes place and a pressure wave, usually called shock wave (SW), that travels into the liquid medium can be observed. This is followed by the evolution of a cavitation bubble (CB) that persists in the liquid buffer for many tens of microseconds with an oscillating dynamics that can be repeated different times [11, 12]. Since these processes occur on a short timescale and in small volume, many aspects of the LAL mechanism have not been definitively clarified. It has been generally accepted that the laser-induced CB plays a key role in NP generation, but the detailed mechanism of NP formation and growth is still a matter of debate. It has been shown that NP formation takes place inside the CB, and that smaller NPs are transported towards the liquid environment, whereas secondary larger NPs are trapped inside the CB until its collapse [13–15]. On the other hand, it has been suggested that the high pressure and high temperature suffered by the target during the CB collapsing phase can induce a plasma-related ablation mechanism [16, 17].
Among the experimental parameters that can be varied during laser ablation, it has been proven that laser pulse length is strategic in determining the ablation mechanism, and consequently the physical and chemical properties of materials synthesized by LAL. Although most LAL experiments are performed using nanosecond laser pulses, it has been shown that the use of ultrafast sources, such as femtosecond ones, enables the possibility of obtaining particles in unusual phases such as nanodiamonds [18] or rutile NPs [19].
The laser ablation in liquid of a GaAs bulk target has been achieved by nanosecond and picoseconds laser sources. Since 2005 Lalayan has reported the study of the luminescence properties of GaAs quantum dots obtained by LAL in ethanol, acetone, and distilled water [20], without any structural and chemical characterization of the obtained NPs. On the other hand, many authors have observed that by the short pulse laser ablation of GaAs in organic or aqueous solvents, gallium-rich NPs are formed [21–24]. The gallium excess is related to the amorphous component in the produced NPs [22]. In order to improve the stability of the colloidal solution, the coating GaAs nanocrystals with a SiO2 thin film has been reported by Abderrafi et al [24].
With the aim of highlighting the role of pulse duration on the ablation mechanism and the properties of the obtained nanostructures, we report the laser ablation of GaAs in acetone, using laser sources with nanosecond and femtosecond pulse lengths. To the best of our knowledge this is the first study on the ablation in liquid of a GaAs target performed by an ultrashort laser source.
2. Experimental
The laser ablation experiments were performed by two laser sources: Nd:YAG ( = 532 nm,
= 532 nm,  = 7 ns, 10 Hz repetition rate) and Nd:glass (
= 7 ns, 10 Hz repetition rate) and Nd:glass ( = 527 nm,
= 527 nm,  = 250 fs, 10 Hz repetition rate). The laser beams were focused perpendicular to the surface of the target through the liquid surface by a 40 mm focal length plano-convex lens mounted on a micrometric translation stage. The laser energy reaching the target surface was 30 mJ and 3 mJ, for a fluence of 30 J cm−2 and 20 J cm−2 for the short and ultrashort laser pulses, respectively. The two laser sources have quite similar wavelengths and the same repetition rate. Also, the fluencies used in the nanosecond and femtosecond ablation experiments are comparable, so that the observed differences in the ablation dynamics and NP properties can only be ruled out to the effect of the different pulse duration. A GaAs (1 0 0) monocrystal (Aldrich, purity 99.9%, 0.5 mm thick) was used as the target. It was contained in a 2 cm × 5 cm quartz cuvette and covered by a 2 cm column of liquid in order to avoid the evaporative explosion of the liquid surface layer. Acetone (Aldrich, 99% purity) was used as the liquid medium.
= 250 fs, 10 Hz repetition rate). The laser beams were focused perpendicular to the surface of the target through the liquid surface by a 40 mm focal length plano-convex lens mounted on a micrometric translation stage. The laser energy reaching the target surface was 30 mJ and 3 mJ, for a fluence of 30 J cm−2 and 20 J cm−2 for the short and ultrashort laser pulses, respectively. The two laser sources have quite similar wavelengths and the same repetition rate. Also, the fluencies used in the nanosecond and femtosecond ablation experiments are comparable, so that the observed differences in the ablation dynamics and NP properties can only be ruled out to the effect of the different pulse duration. A GaAs (1 0 0) monocrystal (Aldrich, purity 99.9%, 0.5 mm thick) was used as the target. It was contained in a 2 cm × 5 cm quartz cuvette and covered by a 2 cm column of liquid in order to avoid the evaporative explosion of the liquid surface layer. Acetone (Aldrich, 99% purity) was used as the liquid medium.
The fast shadowgraphic set-up used to characterize the SWs and CBs has been fully described elsewhere [12]. The shadowgraph images were obtained using a He–Ne laser source as the background light source, and the laser beam was expanded using a 10X microscope objective. The images were collected by means of a fast ICCD detection system (PI Max II, 1024 × 1024 pixel) and the ICCD gate-width was varied from 200 ns to 10 μs.
A high-resolution TEM (HRTEM) FEI-TECNAI G2 20 TWIN, operating at 200 kV was used to study both the dimensions and distribution of the NPs obtained by the laser ablation. To this aim, some drops of a sample solution were placed on a holey carbon-coated copper grid.
The EDX analysis was performed using an ESEM XL30-FEI instrument. For the microRaman measurements a HORIBA LabRam 800 HR apparatus equipped with an edge filter, a He–Ne laser ( = 632.8 nm), and an Olympus microscope (objectives: 10×/50×/100×) was employed. The laser spot size impinging on the samples surface was about 10 μm in diameter when the 100x microscope objective was used. A spectral resolution of about 4 cm−1 was obtained by a holographic grating with 600 lines mm−1.
= 632.8 nm), and an Olympus microscope (objectives: 10×/50×/100×) was employed. The laser spot size impinging on the samples surface was about 10 μm in diameter when the 100x microscope objective was used. A spectral resolution of about 4 cm−1 was obtained by a holographic grating with 600 lines mm−1.
3. Results and discussion
During the LAL experiments, the expansion of the laser-induced plasma formed at the solid–liquid interface is strongly confined and delayed. The species present in the supersaturated plasma interact each other in a process of nucleation and growth where free atoms condense on the nuclei, and the nuclei can coalesce originating particles with a polycrystalline structure. The large amount of energy transferred at the solid target surface induces an increase in pressure and temperature at the solid–liquid interface. The target can release the excess of energy by the emission of an intense pressure wave (SW) that expands in the liquid at a velocity comparable to the speed of sound in that medium. At a time delay of 200 ns and 100 ns from the nanosecond and femtosecond laser pulses, respectively, the generation of a hemi-spherical SW was observed on the target surface that travels in the liquid at a constant propagation rate. In the time range of measurements used here, the SWs have a propagation rate of 1173 ± 10 m s−1, irrespective of the laser pulse length used. This propagation rate is comparable to the speed of sound in acetone (1174 m s−1).
The pressure gradient and the energy transfer from plasma to liquid, with the formation of a vapor layer around the plasma volume, induce the formation of bubbles, usually called CBs. It has been shown that the dynamics of a CB includes an expansion stage with subsequent shrinking. At the beginning of the oscillation period the pressure inside the bubble is larger than the pressure of the surrounding liquid, and the bubble grows until the pressure inside the bubble reaches a minimum value. Under these conditions, the bubble starts to shrink due to the pressure of the liquid medium, and finally collapses compressing the bubble's contents near the surface of the target and releasing a substantial amount of energy to the target surface [25]. The contents of the bubble acts as a piston and causes the rebound of the bubble itself in an oscillating behavior that can be repeated several times, with a dynamics that follows the well-known Rayleigh–Plesset model [26].
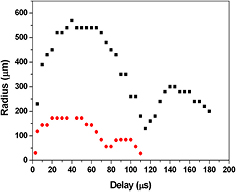
In figure 1 the shadowgraphic images of a CB obtained by the nanosecond laser ablation of GaAs in acetone are reported. In figure 2 the evolution of the CB radius with time observed during the nanosecond and femtosecond laser ablation of GaAs in acetone is compared. The nanosecond-induced bubble expands in the liquid, reaches its maximum radius of 540 μm after a delay of 56 μs after the laser shot, and then collapses (after a delay of 128 μs) compressing the bubble's contents near the target surface. During the shrinking phase, the bubble presents an asymmetric shape, probably due to the pressure of the surrounding liquid. After the bubble collapses a second oscillation begins and the ejection of material can be clearly observed. It has been shown by scattering experiments [25] that the ejected material usually consists of particles condensed inside the CB. On the other hand, the femtosecond-induced CB presents a smaller radius and lifetime (180 μm and 75 μs, respectively) with respect to the nanosecond-induced one. No ejection of material after the bubble collapse is observed, probably due to the small dimensions of the CB. The dependence of the CB dimension on the laser pulse duration was investigated previously. In particular, the large CB induced during the nanosecond laser ablation is related to the higher energy conversion to bubble energy for the nanosecond pulse with respect to the shorter pulses and/or to the high kinetic energy of the atoms in the plume due to the absorption of the trailing part of the ns laser pulse [27, 28].
Figure 1. Shadowgraph images observed at various delay times after the irradiation of the nanosecond laser onto a GaAs target immersed in acetone. The acquisition gate was set at 1 μs; (a) 20 μs; (b) 45 μs; (c) 115 μs; (d) 145 μs; (e) 190 μs.
Download figure:
Standard image High-resolution imageFigure 2. Temporal variation of the CB radius induced by nanosecond (■) and femtosecond ( ) laser ablation of GaAs in acetone.
) laser ablation of GaAs in acetone.
Download figure:
Standard image High-resolution imageWe evaluate that the differences observed in the CB dynamics induced by laser sources operating in short and ultrashort temporal regimes can influence the characteristics of the forming particles.
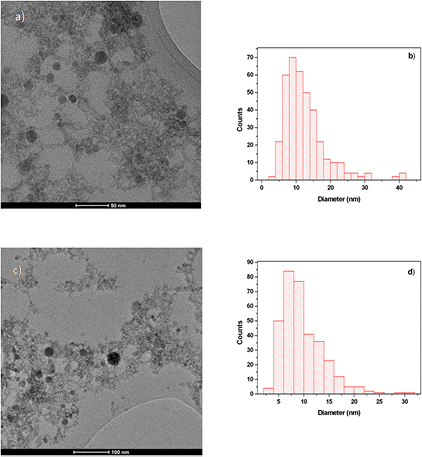
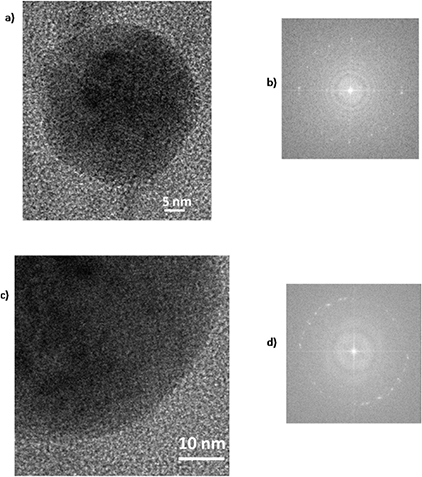
In figure 3 the TEM images and the relative size dispersion of the NPs produced in acetone by nanosecond and femtosecond ablation are shown. The obtained NPs are predominantly spherical in shape and with a mean diameter of 13 nm and 9 nm, respectively. The typical bi-modal size distribution of the particles' diameter, usually produced by femtosecond LAL [12, 29], was not been observed in our experiments and the particles obtained by ablation in both temporal regimes present lognormal size distributions. The condensation of the grains during the particles' formation led to the polycrystalline structural characteristics of the nanomaterials achieved by laser ablation in liquid. The HR-TEM images (figure 4) of the particles obtained by the nanosecond and femtosecond laser sources show that the obtained NPs are composed of nanometric crystalline domains. The bright polycrystalline diffraction spots present in the FFT images allow us to evaluate a lattice spacing of 0.33 nm. This value is in good agreement with the lattice spacing of the (1 1 1) planes in crystalline cubic GaAs [23, 24]. Moreover, it is possible to observe that the particles obtained by nanosecond ablation contain additional amorphous domains.
Figure 3. TEM image and the corresponding size distribution of GaAs NPs obtained by laser ablation in acetone by (a), (b) nanosecond and (c), (d) femtosecond laser sources.
Download figure:
Standard image High-resolution imageFigure 4. HRTEM and 2D FFT of a single nanoparticle obtained by (a), (b) nanosecond and (c), (d) femtosecond laser ablation of GaAs in acetone.
Download figure:
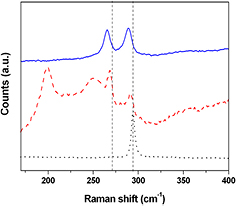
Standard image High-resolution imageIn figure 5 the microRaman spectra of the particles obtained in both ablation conditions, together with the Raman spectrum of the GaAs target, are presented. The Raman spectrum of the GaAs target presents the transverse optical (TO) and longitudinal optical (LO) narrow bands at 270 cm−1 and 294 cm−1, respectively. The observed relative intensity of the TO and LO peaks is characteristic of high quality GaAs crystals with (1 0 0) orientation [7, 30]. Both the TO and LO phonon modes of crystalline GaAs can be observed in the Raman spectra of all the samples. These modes are shifted towards lower energies and are asymmetrically broadened. Both effects can be related to the confinement of the phonon modes in the NPs and to the presence of defects in the GaAs crystals [31, 32]. The Raman spectra of the NPs obtained by femtosecond laser ablation show only TO and LO modes at 265 cm−1 and 289 cm−1, respectively. For the samples prepared with the nanosecond laser source we observe Raman peaks corresponding to TO and LO modes at wavenumbers of 268 cm−1 and 291 cm−1, respectively. In addition, two new signals on the low-energy side can be observed, at 199 cm−1 and 250 cm−1, respectively. The peak at 199 cm−1 can be associated with a libration–translation mode of Ga2O3, as reported by Kumar et al [33]. Because of the large width and the high frequency shift, the broad band centered at 250 cm−1 cannot be associated with the GaAs TO or LO modes. This band is usually related to the existence of amorphous GaAs associated with arsenic vacancies [31, 32, 34]. No signals related to arsenic oxide are observed in this spectral range. The EDX analysis confirms that the samples obtained by nanosecond ablation show a slight excess of gallium with respect to arsenic (Ga : As = 1.4). On the contrary, the nanoparticles obtained by femtosecond laser ablation present a Ga/As ratio of 0.9. The observed intensity ratio of the TO and LO modes in the Raman spectra of all the samples confirm that the crystalline component of the produced NPs does not retain the target crystal orientation and does not have any preferred orientation.
Figure 5. Raman spectra of GaAs (1 0 0) crystal (black dotted line), NPs obtained by nanosecond (red dashed line), and femtosecond (blue line) laser ablation.
Download figure:
Standard image High-resolution imageThe difference in the size distribution and composition observed in the nanomaterials obtained during the ablation of GaAs in acetone with nanosecond and femtosecond lasers pulses can be explained considering the different ablation mechanisms occurring in the two cases. The laser ablation mechanisms in the nanosecond and femtosecond regimes in vacuum or in the presence of a buffer gas [35–37] and, recently, also in the presence of a liquid confinement [25, 38], have been widely investigated. When a nanosecond pulse hits a metallic or semiconducting target, the reached intense heating of the target surface leads to its melting and evaporation that occur at the beginning of the laser pulse. When the ablation is performed in vacuum, during the incidence of the laser pulse, the isothermal expanding plasma can be further augmented at its inner surface with the evaporated material from the target, since the high expansion rate of the plasma edge makes the plasma transparent to the impinging laser beam [35]. On the contrary, for ablation performed in dense media, the plasma expansion is hindered by the consequent absorption of the trailing part of the laser pulse [28]. The evaporated material is further heated by the absorption of the laser beam with the formation of a high-temperature plasma made of ions, atoms, electrons, and droplets. During their permanence inside the laser-induced plasma, the melted drops ejected from the target can be vaporized [10] and, if the target is composed of elements with different vapor pressures, a non-congruent vaporization can occur [39]. Considering that at temperatures higher than 950 K the equilibrium vapor pressure of As is higher than that of Ga [40], the loss of the most volatile component could modify the particles' composition due to the preferential evaporation of As. During LAL, the atoms, ions, and clusters forming the plasma are confined inside the CB and can coalesce the originating NPs that can aggregate during the shrinking phase of the CB [10]. The high-temperature, high-pressure conditions of the laser-induced plasma could induce the excitation and eventually the dissociation of the liquid molecules at the plasma–liquid interface, and high-temperature chemical reaction between the species from the laser-induced plasma and the liquid molecules have to be considered. In these experimental conditions, the excess of metallic gallium can be oxidized by the species generated by the acetone dissociation and/or by the atmospheric oxygen dissolved in the liquid medium [10] originating Ga2O3, whose signals are observed in the Raman spectrum of the particles obtained by nanosecond ablation.
On the other hand, the femtosecond pulse duration is shorter than the full ablation process. Generally, this involves, first, the excitation of the material thin layer electrons by laser photon absorption, then the transfer of the laser energy to the lattice results in a very fast heating of the absorbing volume, which melts with no significant expansion of volume. Finally, a fast adiabatic expansion of the overheated liquid occurs, by the well-known 'phase explosion' mechanism, and the ejection of atoms, small clusters, and NPs can be observed [41, 42]. Unlike the nanosecond pulse-induced process, the ablated species formed by the femtosecond laser sources do not interact with the laser pulse itself. It has been proposed that the main effect of the liquid medium is to confine the heated material and reduce the phase explosion mechanism, limiting the ejection of a large cluster [43]. The SEM investigation of a metallic target exposed to femtosecond irradiation in a liquid environment shows that mushroom-like structures are formed. These structures are justified considering the expulsion of a molten layer [44]. Then, the rapid cooling down of the ejected material, due to the liquid confinement, allows the formation of particles that can retain the target stoichiometry.
The CB formed during the femtosecond laser ablation of GaAs in acetone, as a consequence of the energy transfer from the plasma to the liquid, is characterized by low dimensions and lifetime. In these experimental conditions, the possibility that polycrystalline NPs can grow inside the CB is reduced. Also, the formation of larger particles, usually related to the melting effect due to the collapse of the CB, is hindered. Consequently, the bimodal size distribution often observed in femtosecond LAL was not observed in the experimental conditions here reported.
4. Conclusion
We have studied and compared the nanosecond and femtosecond laser ablation of GaAs in acetone. The use of laser sources with nanosecond and femtosecond pulse temporal lengths allows us to obtain nanomaterial characterized by its peculiar composition and morphology, due to the different ablation mechanism in the two temporal regimes. Nanosecond laser ablation allows us to obtain quite spherical nanoparticles characterized by a Ga/As ratio of 1.4, where the gallium excess is present as gallium oxide. A certain amount of amorphous gallium arsenide is present, as shown by microRaman analysis. On the other hand, by femtosecond LAL a stoichiometric polycrystalline and low dispersed GaAs NPs can be obtained. We suggest that the presence of gallium oxide and amorphous gallium arsenide observed in NPs obtained by nanosecond laser ablation can be referred to as the melting effect typical of the ablation performed by nanosecond laser sources. The gallium excess can be justified considering the different vapor pressure of gallium with respect to arsenic at the temperature reached inside the CB, where the NPs are formed and grow. So, the use of ultrashort laser sources should be preferred to obtain nanostructures that retain the target stoichiometry.