ABSTRACT
This contribution is focused on the concurrent pathway to the Strecker synthesis of amino acids in an astrophysical-like environment. We indeed use experimental and modeling simulations to investigate the possibility to form the aminomethanol (HOCH2NH2) in concurrence with the hydroxyacetonitrile (HOCH2CN) from ices containing at 40 K formaldehyde (CH2O), ammonia (NH3), and cyanide ion (CN−). We demonstrate using infrared spectroscopy and mass spectrometry that the formation of the aminomethanol (Ea = 4.5 kJ mol−1) is competing with the hydroxyacetonitrile formation (Ea = 3.9 kJ mol−1). The ratio between aminomethanol and hydroxyacetonitrile depends on the initial ratio in the ice between ammonia and cyanide. An increase of cyanide ion provides a decrease in aminomethanol formation. Since the aminomethanol is the first step through the formation of glycine in astrophysical environments, these data are important for understanding the possibility of forming glycine in such environments. Furthermore, using a reduced kinetic model, we evaluate the astrophysical environments in which the aminomethanol and hydroxyacetonitrile can be formed and evolved without degradation. The results suggest that these two molecules could be formed in molecular clouds or protostellar disks, and subsequently incorporated inside comets or asteroids. Therefore, hydroxyacetonitrile and aminomethanol could be formed before the formation of the solar system, which suggests that hydroxyacids and amino acids, such as those detected inside meteorites, have been formed in various astrophysical environments.
Export citation and abstract BibTeX RIS
1. INTRODUCTION
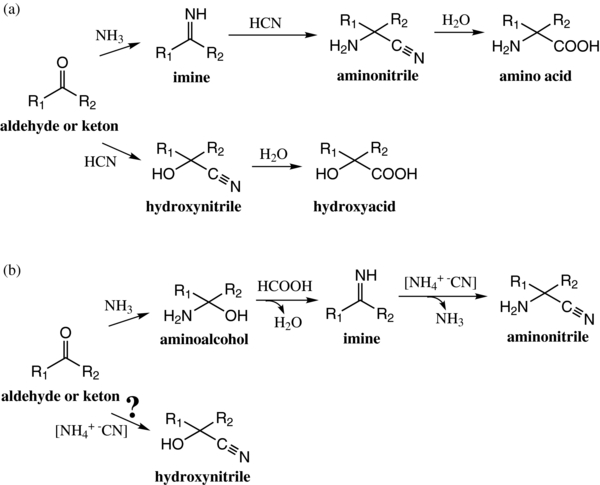
Amino acids are building blocks of fundamental biomolecules, which explains that the search for them in astrophysical environments is one of the main objectives in seeking clues for life in such environments. Only the simplest amino acid, the glycine, has been tentatively observed in hot molecular cores (Kuan et al. 2003), but its detection is still debated (Snyder et al. 2005). The Stardust mission, which flies by the comet Temple-1 has provided a glycine detection from terrestrial analyses of returned samples (Elsila et al. 2009). Furthermore, various meteorite analyses have shown the presence of a high amino acid variety (up to 80 amino acids), which for a large part come from the soluble part of the organic matter of such objects (Cronin et al. 1994; Cronin & Pizzarello 1983). Therefore, it seems that amino acids could be formed in various astrophysical environments, but it is obvious that their presence in these environments does not imply the presence of past or extant life. These results show at most that the exogenous organic matter can be considered as a starting material for the development of a prebiotic chemistry in specific environments such as the primitive Earth was. However, some laboratory experiments have shown that amino acids are not really stable in astrophysical-like conditions. Therefore, rather than free amino acids, these compounds could be present in astrophysical environments through their precursors, which will lead to amino acids after chemical treatment such as hydrolysis. For instance, glycine (NH2CH2COOH) is photolyzed five times faster than its corresponding nitrile precursor, the aminoacetonitrile (NH2CH2CN) (Bernstein et al. 2004). Since aminonitrile compounds are more stable than amino acids in astrophysical conditions, they are considered as a possible reservoir of amino acids (Bernstein et al. 2004). The aminoacetonitrile, the glycine precursor, has indeed been detected in SgrB2 (Belloche et al. 2008). Its pathways of formation are still under investigation, but some results have shown that it could be formed from vacuum ultraviolet (VUV) irradiation of ammonia/acetonitrile ices (Danger et al. 2011b), or at the surface of interstellar or nebular grains through the Strecker synthesis (Danger et al. 2011a). The Strecker synthesis (Strecker 1854) is also presented as one of the main pathways for the formation of amino acids that are detected in the soluble organic fraction of meteorites (Cronin et al. 1994; Elsila et al. 2007; Pizzarello et al. 2010). In the case of meteoritic amino acids, the Strecker synthesis should occur inside asteroids through a liquid phase, providing the right chemical conditions (Figure 1(a)). In such liquid phase, the reaction of ammonia on aldehyde or ketone precursors leads to imine formation, which forms aminonitrile in the presence of hydrogen cyanide. Due to the presence of water inside asteroids, these aminonitrile compounds can then be hydrolyzed, leading to amino acid formation. Amino acid formation from the Strecker synthesis inside asteroids is also strengthened by the detection in meteorites of hydroxyacid derivatives (R1R2C(OH)COOH) (Cronin et al. 1994; Pizzarello et al. 2010) and iminoacid derivatives (R1R2(NHR3)COOH) (Lerner & Cooper 2005). Hydroxyacid compounds are indeed formed in aqueous solution from a chemical pathway concurrent to the Strecker reaction, as shown in Figure 1(a) (Taillades et al. 1998). Their formation comes from the direct reaction of hydrogen cyanide to aldehyde or ketone derivatives, leading to hydroxynitrile derivatives. Their subsequent hydrolysis provides hydroxyacid derivatives.
Figure 1. (a) Experimental pathways for amino acid formation in liquid water via the Strecker synthesis (ammonia NH3 pathway) and its concurrent reaction, the hydroxyacid formation via the direct reaction of hydrogen cyanide with aldehyde or ketone derivatives (hydrogen cyanide HCN pathway). (b) Experimental pathways in astrophysical-like conditions for aminoacetonitrile formation in the solid phase via the Strecker synthesis (ammonia NH3 pathway) and its concurrent reaction, the hydroxynitrile formation via the direct reaction of ammonium cyanide with aldehyde or ketone derivatives (ammonium cyanide [NH+4 −CN] pathway).
Download figure:
Standard image High-resolution imageThe Strecker synthesis is usually considered to occur in liquid aqueous media. However, as previously discussed, the Strecker synthesis has been demonstrated to occur out of liquid water up to the aminonitrile formation (Danger et al. 2011a). This synthesis can indeed occur in the solid phase, in the presence or absence of solid water, under low pressure (10−9 mbar) from 50 K to 210 K depending on the reaction step (Figure 1(b)). These experimental conditions simulate those observed in molecular clouds or in nebulae. In these conditions, the first step consists in the condensation of ammonia with formaldehyde leading to the formation of the corresponding amino alcohol (Bossa et al. 2009; Duvernay et al. 2010; Woon 1999). The amino alcohol is then dehydrated in the presence of an acid (e.g., HCOOH) to produce the corresponding imine (Vinogradoff et al. 2011, 2012). The last step concerns the addition of cyanide onto imine to form the aminoacetonitrile (Figure 1(b); Danger et al. 2011a). Therefore, the Strecker synthesis can occur in various astrophysical environments. The only step that seems to be unlikely out of liquid water is the aminonitrile hydrolysis leading to corresponding amino acids (Borget et al. 2012; Danger et al. 2011a; Rimola et al. 2010). Since, in aqueous media, the hydroxynitrile formation competes with aminonitrile formation (Pizzarello et al. 2010; Taillades et al. 1998), in this contribution we investigate the possibility to form such derivatives in the solid phase with relevant astrophysical conditions, meaning at low temperature (from 40 K to 300 K) and under vacuum (10−9 mbar). In these conditions, since in the absence of acid the first step of the Strecker reaction ends in the aminoalcohol formation, we focus our experimentation on the competition between the hydroxyacetonitrile (HOCH2CN) and the corresponding aminoalcohol, the aminomethanol (HOCH2NH2) (Bossa et al. 2009). It has been shown theoretically that the hydroxyacetonitrile can be formed in ice analogs (Woon 2001). We therefore use ices containing formaldehyde (CH2O), ammonia (NH3), cyanide (CN−), and/or water to estimate the branching ratio between aminomethanol and hydroxyacetonitrile formation according to the initial composition of the ice mixtures. Furthermore, using a simple chemistry model, we are able to suggest a scenario for the formation and evolution of these compounds from molecular clouds to their incorporation inside comets or asteroids into interplanetary environments. These results are discussed in an astrophysical discussion.
2. EXPERIMENTAL DETAILS
2.1. Operating Systems
Potassium cyanide, formaldehyde, dichloromethane, and potassium hydroxide were purchased from Sigma Aldrich, stearic acid from Fluka analytical, and ammonia (99.9% purity) from Air Liquide. Dichloromethane was distilled over phosphorus pentoxide. Reagents were mixed in different ratios in a Pyrex vacuum line using standard manometric techniques. The gaseous mixture was deposited at a rate of 6 × 10−1 mol min−1 on a gold-plated surface kept at 40 K with a model 21 CTI cold head. The warming up of the samples was performed at a heating rate of 5 K min−1 using a resistive heater along with a Lakeshore model 331 temperature controller. The infrared spectra of the sample were recorded in reflection mode between 4000 and 600 cm−1 using a Nicolet Magna 750 FTIR spectrometer with an MCT detector. Each spectrum was averaged over one hundred scans with 1 cm−1 resolution. The mass spectra were monitored using an RGA quadrupole mass spectrometer (MKS Microvision-IP plus) as the products are desorbed during a controlled temperature ramp. The ionization source is a 70 eV impact electronic source and the mass spectra are recorded between 1 and 70 amu for a full scan, or recorded for specific mass using the SIM (selected ion monitoring) mode. The VUV irradiation source is an H2 flux lamp irradiating in a range from 120 nm until a continuum in the visible through an MgF2 window directly connected on our cryostat. The VUV flux is 2 × 1015 photons cm−2 s1, which is 1012 times more important than the flux of cosmic rays induced by UV photons in the dense molecular clouds of the interstellar medium.
2.2. Product Syntheses
The hydrogen cyanide (HCN) gas used in these experiments was synthesized directly on the Pyrex line at 10−3 mbar using the following protocol (Gerakines et al. 2004). In a manifold, 378 mg of stearic acid (CH3(CH2)16COOH, 1.32 mmol) are mixed with 86 mg of potassium cyanide (KCN, 1.32 mmol). The manifold is then directly connected to the Pyrex line and left under vacuum for 24 hr. HCN is synthesized by slowly heating the manifold including CH3(CH2)16COOH/KCN and cooled with a water bath (room temperature); after that the pressure sought is reached in the Pyrex line. Usually, 5 mbar of pure HCN are synthesized per experiment, which allows five to six experiments per CH3(CH2)16COOH/KCN tube. The hydroxyacetonitrile, also named glycolonitrile, was synthesized following Gaudry (1974). The hydroxyacetonitrile is deposited directly from its synthesis tube. Therefore, the amount deposited can only be estimated from the infrared spectra.
2.3. Infrared Spectroscopy for Product Quantification
The amount of formaldehyde is obtained from the band at 1717 cm−1 (which has a band strength of  = 9.6 × 10−18 cm molecule−1). The amount of NH3 is obtained from the band at 1106 cm−1 (
= 9.6 × 10−18 cm molecule−1). The amount of NH3 is obtained from the band at 1106 cm−1 ( = 1.3 × 10−17 cm molecule−1). For the amount of HCN, we consider that all the HCN is converted in CN−, since when HCN and NH3 in excess are mixed in the same ramp and deposited, only the [NH+4 −CN] salt is formed (Danger et al. 2011a). Therefore, the initial ice can be reduced in our experimental conditions to a CH2O:NH3:[NH+4 −CN] ice. Furthermore, since the CN− band is difficult to integrate, its amount was obtained from the ammonium band at 1460 cm−1 (
= 1.3 × 10−17 cm molecule−1). For the amount of HCN, we consider that all the HCN is converted in CN−, since when HCN and NH3 in excess are mixed in the same ramp and deposited, only the [NH+4 −CN] salt is formed (Danger et al. 2011a). Therefore, the initial ice can be reduced in our experimental conditions to a CH2O:NH3:[NH+4 −CN] ice. Furthermore, since the CN− band is difficult to integrate, its amount was obtained from the ammonium band at 1460 cm−1 ( = 4.4 × 10−17 cm molecule−1). However, as the ammonium band overlaps with the formaldehyde feature (1494 cm−1;
= 4.4 × 10−17 cm molecule−1). However, as the ammonium band overlaps with the formaldehyde feature (1494 cm−1;  = 3.9 × 10−18 cm molecule−1), the entire band was integrated and subtracted from the formaldehyde amount obtained from the band at 1717 cm−1, giving the amount of [NH+4 −CN] salt in the ice. The band strength of the hydroxyacetonitrile can be derived from the amount of hydroxyacetonitrile formed during the warming. The amount of formaldehyde consumed has been calculated between 60 K and 80 K from the decrease of the band located at 1717 cm−1 (
= 3.9 × 10−18 cm molecule−1), the entire band was integrated and subtracted from the formaldehyde amount obtained from the band at 1717 cm−1, giving the amount of [NH+4 −CN] salt in the ice. The band strength of the hydroxyacetonitrile can be derived from the amount of hydroxyacetonitrile formed during the warming. The amount of formaldehyde consumed has been calculated between 60 K and 80 K from the decrease of the band located at 1717 cm−1 ( = 9.6 × 10−18 cm molecule−1). The amount of aminomethanol formed has been calculated from its band at 1008 cm−1 (
= 9.6 × 10−18 cm molecule−1). The amount of aminomethanol formed has been calculated from its band at 1008 cm−1 ( = 3.5 × 10−17 cm molecule−1). By subtracting those two values, the amount of hydroxyacetonitrile formed during the warming is obtained. Those values are reported in Table 1. It is important to note that this band strength can only be used for the hydroxyacetonitrile embedded in ices with similar composition to the one used here. Indeed, when the hydroxyacetonitrile is deposited as a pure ice, all the band strengths present large intensity variations depending on the deposit, and consequently the band strength calculated in this experiment cannot be used.
= 3.5 × 10−17 cm molecule−1). By subtracting those two values, the amount of hydroxyacetonitrile formed during the warming is obtained. Those values are reported in Table 1. It is important to note that this band strength can only be used for the hydroxyacetonitrile embedded in ices with similar composition to the one used here. Indeed, when the hydroxyacetonitrile is deposited as a pure ice, all the band strengths present large intensity variations depending on the deposit, and consequently the band strength calculated in this experiment cannot be used.
Table 1. Positions and Attributions of Infrared Absorption Bands of Products Formed at 205 K after the Warming of an Ice Containing CH2O:NH3:[NH+4 −CN] in a 1.0:0.2:0.1 Ratio with Respect to Water and Deposited at 40 K
| Wavenumber | Identification | Attribution | Band Strength | Ref |
|---|---|---|---|---|
| (cm−1) | (cm molecule−1) | |||
| 3351 | νN-H | HOCH2NH2 | ... | (Bossa et al. 2009) |
| 3265 | νO-H | HOCH2NH2 & HOCH2CN | ... | (Bossa et al. 2009; Jacox 1994; Mielke et al. 1989) |
| 2961 | ν asC-H | HOCH2NH2 & HOCH2CN | ... | (Bossa et al. 2009; Jacox 1994; Mielke et al. 1989) |
| 2891 | νC-H | HOCH2NH2 & HOCH2CN | ... | (Bossa et al. 2009; Jacox 1994; Mielke et al. 1989) |
| 2247 | νC≡N | HOCH2CN | ... | (Jacox 1994; Mielke et al. 1989) |
| 1673 | δOH | HOCH2CN | ... | (Jacox 1994; Mielke et al. 1989) |
| 1600 | δNH2 | HOCH2NH2 | ... | (Bossa et al. 2009) |
| 1468 | δ OH | HOCH2NH2 | ... | (Bossa et al. 2009) |
| 1432 | δCH2 | HOCH2CN | ... | (Jacox 1994; Mielke et al. 1989) |
| 1390 | δCH2 | HOCH2CN | ... | (Jacox 1994; Mielke et al. 1989) |
| 1110 | νC-N+νC-O | HOCH2NH2 | ... | (Bossa et al. 2009) |
| 1052 | νC-O | HOCH2CN | 2.05 × 10−18a | (Jacox 1994; Mielke et al. 1989) |
| 986 | ωCH2 | HOCH2NH2 | 3.5 × 10−17 | (Bossa et al. 2009) |
| 884 | νC-C | HOCH2CN | ... | (Jacox 1994; Mielke et al. 1989) |
Notes. Vibration mode: stretching (ν), bending (δ), rocking (ρ), wagging (ω). Type of vibration mode: asymmetric (as), symmetric (s). Band strengths used for the calculation of column densities are also reported. aFor the calculation of the hydroxyacetonitrile band strength see the text in Section 3.1.
Download table as: ASCIITypeset image
2.4. Reduced Kinetic Model
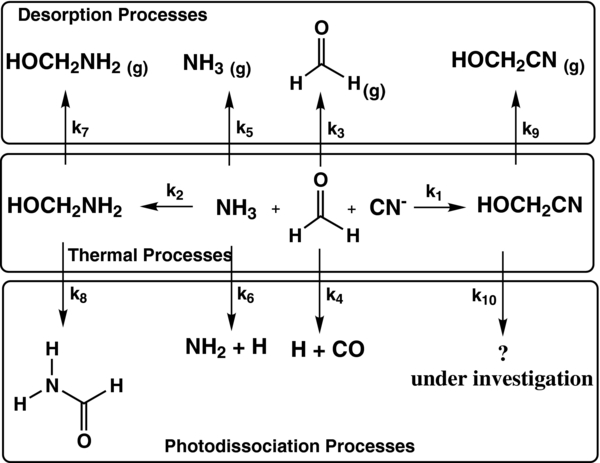
The abundances of aminomethanol and hydroxyacetonitrile have been determined in various environments, such as cold molecular clouds, comets, or young stellar objects, for different ratios of ammonia and cyanide, using a numerical resolution of a system of first-order ordinary differential equations to model the formation and destruction of each species, the water abundance being kept constant (Figure 6 and Equation (1)). These equations can be sorted into three different classes: thermal formation, thermal desorption, and photodestruction. The chemical reaction network and the ordinary differential equation system are shown in Figure 6 and Equation (1). For two-body thermal reactions (k1 and k2) and thermal desorptions (k3, k5, k7, k9), the rate coefficients are given in the form k = Aexp (− E/RT), where T is the ice temperature, A the pre-exponential factor, and E the energy (activation or desorption). Photodissociation rates (k4, k6, k8, k10) are written as k = σf, where σ represents the photodissociation cross-section for a given species and f the ultraviolet radiation field depending on the astrophysical environment (Woodall et al. 2007). Kinetic data are used as well as the photodissociation cross-section obtained in this present work (σ photo (HOCH2CN) = 5.8 × 10−20 photons−1 cm2) to estimate kinetic rates of the formation (k1) and photodissociation reactions (k10) of HOCH2CN. The photodissociation rates of H2CO (k4), NH3 (k6), and HOCH2NH2 (k8) are also taken into account, using the kinetic rates given in the literature. We also included the desorption rate for all the reactants and all the products. All thermal reaction, photodissociation, and desorption rates are given in Table 2:
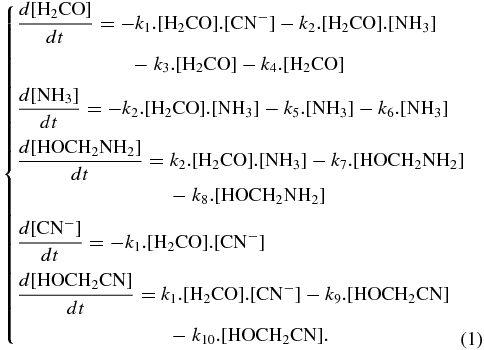
Table 2. Parameters for Thermal Formation, Photodestruction, and Desorption Rates
| Species | Rate Constant | 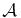 |
E | Ref | |
|---|---|---|---|---|---|
| (s−1) | (kJ mol−1) | ||||
| Thermal formation ratea | HOCH2NH2 | k1 | 0.5 × 10−2 | 4.4 | (Bossa et al. 2009) |
| HOCH2CN | k2 | 2.8 × 10−1 | 3.8 | This work | |
| NH3 | k5 | 3 × 1012 | 25.5 | (Sandford & Allamandola 1993) | |
| Desorption ratea | H2CO | k3 | 3 × 1012 | 20 | Unpublished |
| HOCH2CN | k9 | 3 × 1012 | 58 | c | |
| HOCH2NH2 | k7 | 3 × 1012 | 58 | (Bossa et al. 2009) | |
| Rate Constant | σ (photons−1 cm2) | ||||
| HOCH2CN | k10 | 5.8 × 10−20 | This work | ||
| Photodestruction rateb | NH3 | k6 | 3.2 × 10−20 | (Cottin et al. 2003) | |
| H2CO | k4 | 10−19 | (Woodall et al. 2007) | ||
| HOCH2NH2d | k8 | 4.5 × 10−19 | (Duvernay et al. 2010) | ||
Notes. aThe rate coefficients are given in the form k = Aexp (− E/RT), where T is the ice temperature, A the pre-exponential factor, and E the energy (activation or desorption). bPhotodissociation rates are written as k = σf, where σ represents the photodissociation cross-section and f the interstellar ultraviolet radiation field. cSince HOCH2CN and HOCH2NH2 have the same desorption temperature under our vacuum conditions, we use the same desorption rate. dWe used the experimental value determined for NH2CH(CH3)OH.
Download table as: ASCIITypeset image
3. RESULTS AND DISCUSSION
3.1. HOCH2CN Characterization and Formation from CH2O, HCN, and NH3
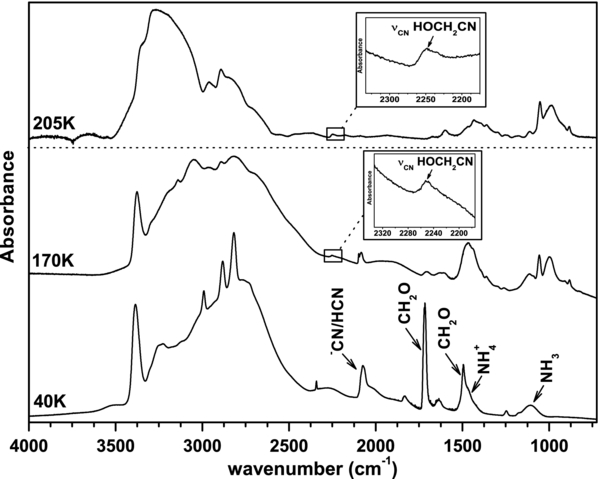
The aminomethanol (HOCH2NH2) is easily formed at 70 K from an ice mixture containing formaldehyde (CH2O), ammonia (NH3), and water (H2O) (Bossa et al. 2009). This reaction is the first step of the Strecker synthesis toward the aminoacetonitrile formation. However, in order to form the aminoacetonitrile, hydrogen cyanide has to be present in the medium. In aqueous solution, it is known that the aminoacetonitrile (NH2CH2CN) formation is counterbalanced by the formation of the hydroxyacetonitrile (HOCH2CN), which is formed by the direct reaction of hydrogen cyanide on formaldehyde (Taillades et al. 1998). Therefore, in order to estimate the possibility of forming the aminoacetonitrile in astrophysical-like conditions, it is important to investigate the possibility of the competition between the aminomethanol and the hydroxyacetonitrile formation (Figure 1(b)). For that purpose, we deposit at 40 K an ice mixture containing CH2O:NH3:[NH+4 −CN]. NH3 and HCN are mixed in the same vacuum line and then co-deposited with CH2O (see the experimental section for details). Figure 2 displays one of these ices containing CH2O:NH3:[NH+4 −CN] with a ratio 1.0:0.2:0.1. At 40 K, the infrared spectrum is the sum of the individual spectra of the starting materials, indicating the absence of reactivity at this temperature. This ice formed at 40 K is then warmed to 300 K with a temperature ramp of 5 K min−1. Whereas bands of CH2O (1717 cm−1) and of [NH+4 −CN] (2087 cm−1) decrease to their sublimation at 110 K and 160 K, respectively, some new bands appear at 884 cm−1, 986 cm−1, 1052 cm−1, 1110 cm−1, 1390 cm−1, 1432 cm−1, 1600 cm−1, 1673 cm−1, and 2247 cm−1 (Figure 2, 170 K).
Figure 2. Infrared spectra of CH2O:NH3:[NH+4 −CN] in a 1.0:0.2:0.1 ratio deposited at 40 K, at 170 K after the warming of the initial ice at 5 K min−1, and at 205 K at the same warming rate.
Download figure:
Standard image High-resolution imageAt 205 K, only the new infrared bands relative to new products remain (Figure 2). Some of these infrared features are related to aminomethanol (986, 1110, 1432, and 1600 cm−1) (Bossa et al. 2009) and hydroxyacetonitrile (884, 1052, 1673, and 2247 cm−1) as displayed in Table 1, and as shown by the dashed and dotted lines in Figure 3(a) for HOCH2NH2 and HOCH2CN, respectively. Infrared analysis at solid state cannot be directly reduced to the sum of the spectra of individual components. In order to strengthen the presence of aminomethanol and hydroxyacetonitrile in the solid, we perform a complementary analysis using mass spectrometry analysis. The mass spectrum (Figure 3(b)) is monitored at 214 K when both HOCH2NH2 and HOCH2CN sublimate. On this spectrum, two different mass patterns are present: one between m/z = 54 and 57, and another one between m/z = 45 and 47. The comparison of the relative intensity of these patterns with the ones of pure HOCH2CN and pure HOCH2NH2 (Figure 3(b)) confirms the previous infrared attributions, since m/z = 54, 55, 56, and 57 are related to the fragment pattern of HOCH2CN, and m/z = 46, and 47 to that of HOCH2NH2 (Figure 3(b)).
Figure 3. (a) Infrared spectra of pure hydroxyacetonitrile, HOCH2CN, deposited at 40 K, of pure aminomethanol, HOCH2NH2, obtained at 200 K (Bossa et al. 2009), and of a residue coming from the warming of an ice mixture containing at 40 K H2O:NH3:[NH+4 −CN] and subsequently warmed at 5 K min−1 to 205 K. The dashed lines refer to HOCH2NH2 features, and dotted lines refer to HOCH2CN ones. (b) Mass spectra (70 eV electronic ionization) presenting the relative intensity of pure hydroxyacetonitrile, HOCH2CN, obtained at 226 K, of pure aminomethanol, HOCH2NH2, obtained at 200 K, and fragments of desorbing species obtained at 214 K coming from an initial ice containing H2O:NH3:[NH+4 −CN]. M refers to hydroxyacetonitrile fragments, while N refers to aminomethanol ones.
Download figure:
Standard image High-resolution imageThose results demonstrate that from an H2O:NH3:[NH+4 −CN] ice mixture, both aminomethanol and hydroxyacetonitrile are formed (Figure 4). However the ratio between them is directly connected to the initial composition of the ice mixture, and more precisely to the initial NH3/CN− ratio. Indeed from an ice mixture containing H2O:NH3:[NH+4 −CN] in proportion 1.0:0.2:0.1, the branching ratio has been estimated to be 87% in favor of the hydroxyacetonitrile (see the experimental section). When the amount of ammonia is decreased in the initial ice, as for an ice mixture containing H2O:NH3:[NH+4 −CN] in proportion 1.0:0.1:0.2, the reaction evolves in favor of the hydroxyacetonitrile giving a branching ratio of 97% for the hydroxyacetonitrile. Therefore, the branching ratio between hydroxyacetonitrile and aminomethanol effectively depends on the relative amount of [NH+4 −CN] salt compared to ammonia in the initial ice (Figure 4).
Figure 4. Chemical pathways occurring during the warming of an ice containing at 40 K CH2O/CN−/NH3. The ratio between the aminomethanol and hydroxyacetonitrile amounts depends on the initial ratio in the ice between ammonia, NH3, and ammonium cyanide, [NH+4 −CN].
Download figure:
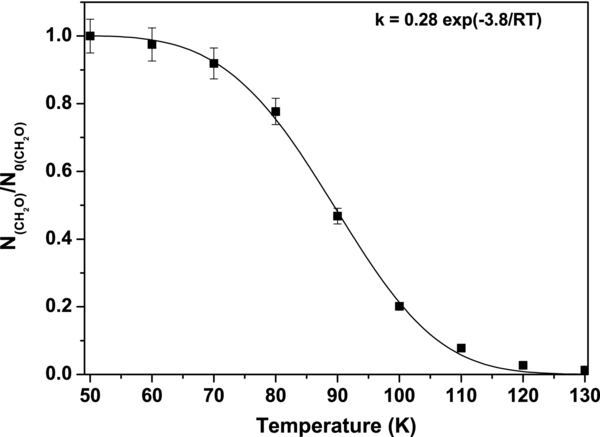
Standard image High-resolution imageThe activation energy for the hydroxyacetonitrile formation has been estimated from an ice mixture containing only traces of free ammonia. Consequently, it can be assumed that, during the ice warming, the formaldehyde will only be consumed by hydroxyacetonitrile formation. This ice has been warmed at 5 K min−1, and the evolution of the formaldehyde amount has been followed using its band at 1717 cm−1 ( = 9.6 × 10−18 cm molecule−1). By tracing this evolution as a function of the temperature (Figure 5) and fitting using Equation (2), the activation energy for the hydroxyacetonitrile formation has been obtained. The corresponding value is 3.86 kJ mol−1. This value is lower than that obtained for the aminomethanol, which is 4.5 kJ mol−1 (Bossa et al. 2009), meaning that in our experimental conditions hydroxyacetonitrile formation is favored over aminomethanol:
= 9.6 × 10−18 cm molecule−1). By tracing this evolution as a function of the temperature (Figure 5) and fitting using Equation (2), the activation energy for the hydroxyacetonitrile formation has been obtained. The corresponding value is 3.86 kJ mol−1. This value is lower than that obtained for the aminomethanol, which is 4.5 kJ mol−1 (Bossa et al. 2009), meaning that in our experimental conditions hydroxyacetonitrile formation is favored over aminomethanol:
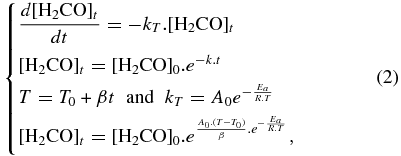
with T being temperature (K), Ea the activation energy (kJ mol−1), A0 the pre-exponential factor (s−1), β the ramp rate (K s−1), and the gas constant R = 8.31 J K−1 mol−1.
Figure 5. Evolution of the formaldehyde amount during a temperature ramp. Assuming that no aminomethanol is formed initially, we derive the activation energy by fitting the experimental data with Equation (2).
Download figure:
Standard image High-resolution image3.2. Astrophysical Discussion
In order to estimate the efficiency of the aminonitrile formation through the Strecker reaction, it is important to take into account the concurrent reactions that could occur (Taillades et al. 1998). This is particularly important for the first step of the Strecker reaction in the condensed phase, which concerns the formation of aminoalcohols from aldehydes/ketones and ammonia (Figure 1(b)). In the general scheme of the Strecker reaction, the starting material includes aldehydes/ketones, ammonia, and hydrogen cyanide. Consequently, the condensation of ammonia with aldehyde or ketone is in direct competition with the condensation of hydrogen cyanide with aldehyde. Therefore, aminoalcohol formation occurs with hydroxynitrile formation (Figure 4).
In this contribution, we used formaldehyde, ammonia, and hydrogen cyanide, three molecules detected in various astrophysical environments, and clearly demonstrated that aminomethanol formation with hydroxyacetonitrile formation. We have also tested these reactions in the presence of a small amount of water, and the same reaction products were obtained. Activation energies of formation of these two compounds are relatively similar (Table 2: 3.8 kJ mol−1 for the hydroxyacetonitrile and 4.4 kJ mol−1 for the aminomethanol; Bossa et al. 2009; Duvernay et al. 2010). This implies that the ratio between the aminomethanol and hydroxyacetonitrile formed will be mainly determined by the initial ratio of the amount of ammonia to cyanide. Consequently, since the formation of the aminoacetonitrile depends on the remaining cyanide in the solid phase (Figure 1(b), reaction of cyanide with imine), it is important to understand the influence of the initial ratio between ammonia and cyanide on hydroxyacetonitrile and aminomethanol formation. In order to estimate the evolution of the relative abundances of aminomethanol and hydroxyacetonitrile in more realistic interstellar ices, we simulate the evolution of the formation of these compounds as a function of time (Figures 6–8) using a restricted chemical system that gives information on the competition between the different processes (experimental section, Equation (1)).
Figure 6. Simplified chemical reaction network used to model HOCH2CN and HOCH2NH2 production.
Download figure:
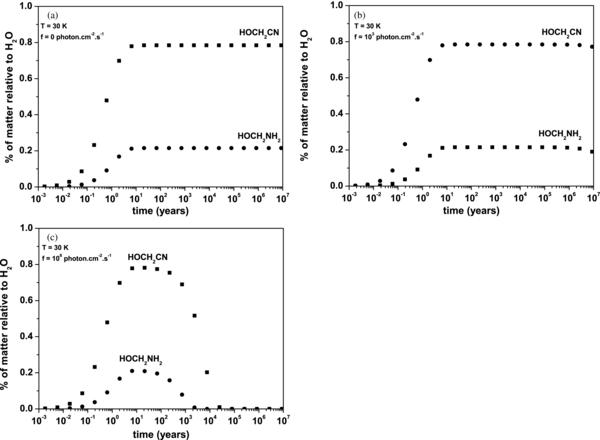
Standard image High-resolution imageFigure 7. Simulation of the evolution of the amount of HOCH2CN and HOCH2NH2 as a function of the time formed from an ice containing NH3, CH2O, and CN− in proportion 10%, 1%, and 1% with respect to water, respectively: (a) displays this evolution at 30 K, (b) the same as (a) with a photon flux of 103 photons cm−2 s−1, and (c) with a photon flux of 108 photons cm−2 s−1. This simulation also takes into account the thermal desorption of HOCH2CN and HOCH2NH2 (Table 2).
Download figure:
Standard image High-resolution imageFigure 8. Simulation of the evolution at 30 K of the amount of HOCH2CN and HOCH2NH2 as a function of the time formed from an ice containing NH3, CH2O, and CN− in proportion 10%, 1%, and 0.1% with respect to water, respectively. This simulation also takes into account the thermal desorption of HOCH2CN and HOCH2NH2 (Table 2).
Download figure:
Standard image High-resolution imageFigure 7(a) displays the evolution of thermal processes leading to the formation of hydroxyacetonitrile and aminomethanol from the initial ice, which contains NH3, H2CO, and CN− in proportion 10%, 1%, and 1% with respect to H2O, respectively, at a constant temperature of 30 K without UV photons. The initial amounts of NH3 and H2CO have been chosen from observational data (Dartois 2005) while we chose the upper limit of detection for CN−. At 30 K, thermal production of hydroxyacetonitrile and aminomethanol begins in less than one year, and reaches a maximum in 10 years. The branching ratio is 80% in favor of the hydroxyacetonitrile, which demonstrates the difference in selectivity between these two compounds, and 0.8% of hydroxyacetonitrile with respect to water is formed. Considering the initial amount of cyanide in the ice (1% with respect to water), the remaining cyanide in the ice is 0.2% with respect to water. Therefore, with this ice mixture, it remains cyanide in order to form the aminoacetonitrile. The total amount of (HOCH2CN + HOCH2NH2) compounds formed represents 1% with respect to water. This implies that all the formaldehyde is consumed, since its initial proportion in the ice is 1%. Furthermore, in a time range coherent with astrophysical environments (up to 107 years), the amounts of HOCH2CN and HOCH2NH2 are constant. Since the thermal desorption is taken into account in the model, this implies that these two compounds remain in the solid phase at the surface of grains.
Figures 7(b) and (c) display the same thermal evolution in the presence of a photon flux of 103 photons cm−2 s−1 (Prasad & Tarafdar 1983) and of 108 photons cm−2 s−1 (Mathis et al. 1983), respectively. Figure 7(b) displays the simulation of the matter evolution inside molecular clouds, whereas Figure 7(c) simulates the evolution that could occur at the edge of molecular clouds, such as in photodissociation regions. In each case, the amount of hydroxyacetonitrile and aminomethanol decreases. Since no thermal desorption has been observed in Figure 7(a), this disappearance is due only to photodegradation processes. In molecular cloud conditions (Figure 7(b)), each compound reaches its maximum of thermal production at the grain surface, and the photodegradation only begins at 3 × 106 years for HOCH2CN and at 106 years for HOCH2NH2. Therefore, in the presence of a low photon flux, the hydroxyacetonitrile and aminomethanol remain stable at the surface of grains. It should be noted that aminomethanol is photodegradated faster than hydroxyacetonitrile. When the photon flux is increased to 108 photons cm−2 s−1 (Figure 7(c)), both compounds also reach their maximum thermal formation after 10 years. The aminomethanol is totally photodegradated after 2200 years, whereas the hydroxyacetonitrile totally disappears from the grain surface after 15,000 years. Consequently, hydroxyacetonitrile and aminomethanol could be detected at the grain surface inside molecular clouds. In photodissociation regions, the photon flux is more important, leading to a rapid photodegradation, which limits their lifetime at the grain surface. Furthermore, in order to define the effect of the initial composition of ices, a simulation is performed with an ice containing at 30 K NH3, CH2O, and CN− in proportion 10%, 1%, and 0.1% with respect to water, respectively. As shown in Figure 8, when the amount of CN− is decreased by a factor of 10, the branching ratio is no longer in favor of the hydroxyacetonitrile, but at 90% favors the aminomethanol. Using these conditions, the formaldehyde is also entirely consumed, since amounts of HOCH2CN and HOCH2NH2 represent 1% of matter relative to water, which is the initial amount of formaldehyde. Furthermore, the amount of hydroxyacetonitrile is 0.1% with respect to water, which means that all the cyanide is consumed. In this environment, there is no cyanide to perform the aminoacetonitrile formation. Another reservoir of cyanide thus has to be taken into account. Consequently, as observed experimentally, the initial ratio in the ice between ammonia and cyanide is the major parameter that balances the ratio between hydroxyacetonitrile and aminomethanol formed at the grain surface. Furthermore, the possibility of forming the aminoacetonitrile in this ice also strongly depends on this initial ratio, since its formation is directly related to the cyanide remaining in the ice after this first reaction step (Danger et al. 2011a).
Figure 9 presents the different environments in which amino acids, hydroxyacids, and their precursors could form. Some hydroxyacids and amino acids detected inside meteorites (Pizzarello et al. 2010) could originate from their grain syntheses inside molecular clouds. Hydroxynitriles and aminoalcohols can also be formed inside comets and asteroids, since formaldehyde, ammonia, and hydrogen cyanide can be incorporated inside such objects when planetary systems are formed. Consequently, hydroxynitriles and aminoalcohols could have been formed in various astrophysical environments showing important variability in temperature, pressure, or hydration conditions. This variability could explain the differences observed in their subsequent products detected in meteorites (Throop 2011), which are hydroxyacids and amino acids (Pizzarello et al. 2010). It is obvious that the Strecker reaction is not the only pathway that leads to the formation and detection of amino acids inside meteorites. The Michael addition has been proposed for the formation of some amino acid derivatives. Further reactions can lead to their formation without the requirement of an aqueous phase, such as the Fischer–Tropsch-type catalysis (Glavin et al. 2010). Furthermore, experimental simulations of interstellar or cometary ices have shown that amino acids could be formed from various chemical pathways (Elsila et al. 2007). This is confirmed by the fact that, depending on the chemical treatment applied to the organic residue obtained at the end of these experiments (Bernstein et al. 2002; Caro et al. 2002), various amounts of amino acids are detected. Before hydrolysis, only small amounts are identified, while after the residue hydrolysis, the quantity of amino acids is much more important (Meinert et al. 2012). The same behavior is observed during meteorite analyses (Glavin et al. 2010). Therefore, some amino acids could be formed directly through reactions like the Strecker synthesis, whereas others could be obtained after the hydrolysis of macromolecules, such as organic residues obtained from experimental simulations of cometary ices, or from the soluble organic matter of meteorites.
Figure 9. Representation of the environments where hydroxynitriles and aminoalcohols can be formed and evolved. (a) In molecular clouds (30 K and low photon flux), competition can occur between hydroxyacetonitrile and aminomethanol formation. The final ratio between these two species will strongly depend on the initial ratio between ammonia and cyanide. (b) When the molecular cloud collapses, some of the remaining grains can aggregate and form interplanetary objects such as comets. Therefore, part of the hydroxyacetonitrile and aminomethanol previously formed is present in these objects. Other compounds such as formaldehyde, ammonia, and cyanide will be also incorporated. Since arise in comet temperature can occur, the formation of hydroxyacetonitrile and aminoacetonitrile from aminomethanol could occur. (c) Finally, in some objects such as asteroids, an aqueous liquid phase could occur, meaning that hydroxyacetonitrile and aminomethanol formed at the surface of interstellar grains and incorporated inside comets will lead to the formation of hydroxyacetic acid and to glycine, respectively. Furthermore, because ammonia, formaldehyde, and cyanide could remain, in this aqueous liquid phase, they could directly form glycine and hydroxyacetic acid.
Download figure:
Standard image High-resolution image4. CONCLUSION
We demonstrated the competing reaction that gives aminomethanol and hydroxyacetonitrile at low temperature (40 K–300 K) and low pressure (10−9 mbar) from an ice mixture containing formaldehyde (CH2O), ammonia (NH3), and ammonium cyanide ([NH+4 −CN]). The branching ratio between these two compounds depends on the initial ratio between the amount of ammonia and ammonium cyanide salt. Ammonia favors the formation of aminomethanol (Ea = 4.5 kJ mol−1), whereas the cyanide salt favors the formation of hydroxyacetonitrile (Ea = 3.9 kJ mol−1). Therefore, as in liquid water, the Strecker synthesis in the solid phase simulating interstellar grains competes with the hydroxynitrile formation. These results are important for further investigations of the possibility to form aminonitriles through the Strecker synthesis at the grain surface in astrophysical-like conditions. Hydroxynitriles as aminoalcohols could be therefore produced at the surfaces of grains and, depending of the local environment, can be incorporated in interplanetary objects such as comets and asteroids. Therefore, the formation of amino acids detected inside meteorites can occur in various astrophysical environments with various degrees of temperature and pressure.
We are indebted to Professor Jean-Claude Guillemin for his advice on the synthesis of the hydroxyacetonitrile. This work has been founded by the French national programme "Physique Chimie du Milieu Interstellaire" (P.C.M.I), "Environnements Planétaires et Origines de la Vie" (E.P.O.V) and the "Centre National des Études Spatiales" (C.N.E.S).