-
PDF
- Split View
-
Views
-
Cite
Cite
Hem Jha, J. S. P. Chaudary, Ramesh Bhatta, Yinghui Miao, Susan Osaki-Holm, Bruce Gaynor, Michael Zegans, Mariko Bird, Elizabeth Yi, Karen Holbrook, John P. Whitcher, Thomas Lietman, Disappearance of Trachoma from Western Nepal, Clinical Infectious Diseases, Volume 35, Issue 6, 15 September 2002, Pages 765–768, https://doi.org/10.1086/342298
Close - Share Icon Share
Abstract
We assessed how much of the observed decline in the prevalence of trachoma in a district of Western Nepal was due to an antibiotic treatment program and how much to an underlying secular trend outside of the program. Although antibiotic treatments clearly have an effect at 6 months, we were unable to show that this effect persisted at 12 months; in fact, long-term gains may be due to a secular trend in the area.
Although trachoma has disappeared from many parts of the world, it still accounts for ∼15% of cases of blindness worldwide [1]. The World Health Organization (WHO) has instituted a trachoma elimination program, with the mission of preventing new cases of blindness from the disease after the year 2020 [2]. A major component of the WHO program is the mass distribution of oral azithromycin to treat the ocular chlamydial infection that causes trachoma [3, 4]. Where antibiotic distributions have been monitored, they have been remarkably successful [5–7]. In some surveys, active trachoma appears not to have returned to pretreatment levels after even a single distribution of azithromycin [6, 7]. However, it is difficult to assess the long-term efficacy of mass treatments, because these reports are often restricted to a small number of villages and have not been adequately controlled for trends other than the trachoma program (i.e., secular trends) or for seasonal effects [8, 9].
These observations have raised several questions. How much of an effect do community antibiotic treatments actually have? Is this effect still present 1 year later? Is active trachoma disappearing even in the absence of specific programs targeting the disease, and, if so, how quickly? In this study we followed the course of clinically active disease in multiple villages in Nepal included in a trachoma-control program. Treatments were administered in different seasons, and some villages were observed for 6-month intervals before treatment. This allowed us to use multivariate analysis to isolate the treatment effect itself, as well as any seasonal effects or underlying secular trends.
Methods. From May 1998 to May 2001, the F. I. Proctor Foundation at the University of California, San Francisco; the Geta Eye Hospital; Helen Keller International; and the Nepal Netra Jyoti Sangh conducted a monitored trachoma program in western Nepal. Twenty-five wards from 3 subdistricts (i.e., village development committees) in the Kailali and Konchapur districts were included in the program. Wards that shared a government elementary school were combined into a single treatment unit, in an effort to avoid the introduction of infection from neighboring areas. Such a combination of wards is called “a village” for the purposes of this study. Research was done within the ethical standards of the institutional review board of the University of California, San Francisco, and the Nepal Netra Jyoti Sangh.
Schedules for community azithromycin treatment were determined according to 2 protocols: (1) a group-randomized trial was used to determine the efficacy of targeting treatment to high-risk groups; in 12 villages, either all children 1–10 years old or children with clinically active trachoma along with all members of their household were treated [7]; and (2) a group-randomized study was used to evaluate seasonal differences; in 6 villages treated in different seasons, all children 1–10 years old were treated. All distributions of azithromycin were in accordance with the current WHO guidelines for antibiotic distribution, which include recommendations for mass treatment in regions with ⩾20% prevalence of clinically active trachoma among children and for either mass treatment or targeted treatment of households with active cases in regions of endemicity with <20% prevalence [3].
Clinical examinations were done by investigators experienced in using the WHO simplified trachoma grading system [10]. A grade of either follicular trachoma or intense trachoma was considered to be clinically active trachoma. A previous study estimated that this trachoma program's coverage of the population was 80% [7]. It is important to note that no other specific trachoma prevention activities (such as hygiene, water-supply cleanliness, or fly-control programs) took place during the course of this study [7].
A multivariate model was used to analyze the prevalence of active trachoma over time among children aged 1–10 years old in the 18 villages [11, 12]. Children in this age group were chosen as the sentinel group, because they are the most likely to have clinically active trachoma [13]. Data from visits to villages where treatment had been administered >1 time or >12 months previously were not included in the analysis; thus, all study data were from visits 6 months before treatment, at the first treatment, 6 months after the first treatment, or 12 months after the first treatment. Covariates included the following: the time since the baseline visit, the season (spring or fall), receipt of treatment 6 months previously, and receipt of treatment 12 months previously. “Spring” was defined as the time from the vernal equinox to the summer solstice, and “fall” was defined as the time from the autumnal equinox to the winter solstice. The logarithm of prevalence was estimated so that the effects of administration of antimicrobial therapy could be described as a percentage reduction (or percentage increase) in prevalence. An autoregression model was used to incorporate correlation within the same village at different time points, and a Gaussian error function was assumed [11]. A second multivariate model was constructed that included terms representing the interaction between season and receipt of antimicrobial treatment. Stata software, version 6 (Stata), was used for statistical analysis.
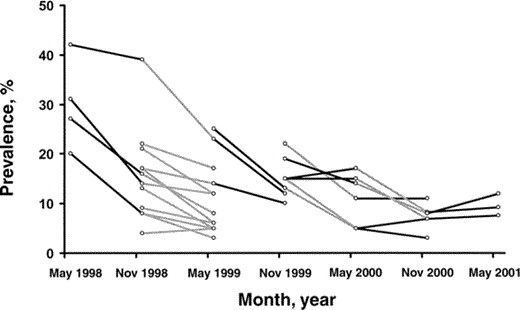
Results. Fifty-two village visits were included in the analysis; 1–3 follow-up visits were made for each village after the baseline visit (figure 1). During each village visit, 180–650 children were examined, and ∼20,000 examinations were performed overall. Before treatment was administered, the prevalence of active trachoma among children 1–10 years old was 4%–39% (mean, 19%). At the final visit, the prevalence had fallen to 3%–17% (mean, 8%). The prevalence of clinically active trachoma decreased by an average of 53% six months after community antibiotic treatment and decreased by 68% twelve months after treatment. Two villages received a second mass azithromycin distribution 1 year after the first. The prevalence progressively decreased 6 and 12 months after the second annual treatment in both villages, reaching an average prevalence of 4%.
Effects of the trachoma-control program in western Nepal. Each time point indicates a visit, and each data point represents the prevalence of active trachoma among children 1–10 years old in a single village at the corresponding visit. Gray lines indicate that antibiotic treatment was administered at the beginning of the 6-month period indicated. Black lines indicate that no antibiotic treatment was administered at the beginning of the 6-month period.
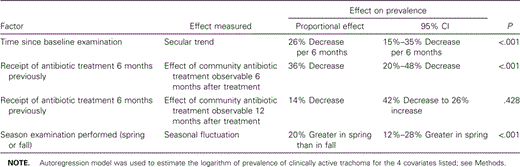
Multivariate analysis revealed that the following 3 covariates were all independently associated with the prevalence of active trachoma at a specific visit: receipt of antibiotic treatment 6 months previously, season of examination, and time since baseline examination (table 1). Receipt of antibiotic treatment 12 months previously was not statistically associated with the prevalence of active trachoma. We were unable to demonstrate that there was a statistically significant interaction between season of examination and receipt of treatment 6 months previously (P = .43) or between season of examination and receipt of treatment 12 months previously (P = .61).
Results of multivariate regression model analysis of the prevalence of clinically active trachoma in children in western Nepalese villages.
Discussion. Several studies have reported success in community distribution of azithromycin for treatment of trachoma. Investigators participating in the Azithromycin in Control of Trachoma Study attributed impressive declines in the prevalence of clinically active trachoma to communitywide administration of antibiotics: they reported a 50% decrease in prevalence among children 15 months after treatment in a single Egyptian village and a 47% decrease in prevalence among all age groups 12 months after treatment in 3 Gambian villages [6, 8, 14]. Prevalence decreased by ∼53% six months and by 43% twelve months after treatment in a single Australian village, a finding that was attributed to targeted household treatment of cases of active trachoma [15].
We had similar success in the trachoma program in western Nepal: we observed a 53% decrease in the prevalence of trachoma 6 months after and a 68% decrease 12 months after the administration of antibiotics. However, after accounting for seasonal variation and an underlying secular trend, we could attribute only an estimated 36% of this decrease to the direct effect of treatment 6 months previously (P < .001; 95% CI, 20%–48% decrease). We were unable to demonstrate that this treatment effect persisted at 12 months; we found a modest and statistically insignificant 14% decline in prevalence that could be attributed to treatment 12 months previously (P = .428; 95% CI, 42% decrease to 26% increase).
Thus, in a geographic area that has no favorable secular trend, we cannot assume that a single mass antibiotic treatment has a long-term effect. In a previous study, a mathematical model of trachoma transmission revealed that multiple periodic treatments can progressively reduce infection in a community, even if the effect of a single treatment wears off over time [4]. Coverage in the Nepal trachoma program was estimated to be only 80% [7]; if the rate of coverage is increased, then the return of infection may be delayed, but the model suggests that multiple treatments would still be necessary for elimination of trachoma [4]. In fact, the WHO currently endorses periodic treatment, although precise guidelines for the intervals between these treatments have not yet been made [3].
The seasonality of trachoma has been thought to be important in many geographic areas, including Morocco, Tunisia, Egypt, and Nepal [16]. However, the magnitude of this seasonal variation has been difficult to assess, because villages typically have not been monitored systematically before administration of antibiotics. We found a 20% (95% CI, 13%–33%) fluctuation in prevalence between the spring and the fall seasons, with the peak prevalence in the spring. It is not clear how to take advantage of this seasonal variation. Is it preferable to administer antibiotics in the peak trachoma season (spring, before the monsoon rains), when there is the most infection, or in the trough season (fall), when programs might have the best chance to locally eradicate infection from households or even small communities? We found no interaction between the effect of treatment and the season in which treatment was administered, suggesting that there is no large advantage to administering antibiotic treatment in a particular season.
Trachoma disappeared from much of Western Europe before the widespread use of antibiotics [17]. Recent reports have indicated that this secular trend continues in other areas now affected by trachoma. One study found that the prevalence of active trachoma had decreased from 66% to 4% between 1959 and 1987 in a single Gambian village. Because there had been only a modest program of antibiotic administration in 1959–1961, the authors attributed the decline to trends independent of the active trachoma program [18]. Similarly, another recent report found that the average prevalence of active trachoma among children in a district in Malawi had decreased from 37% to 14% between 1983 and 1999 in the absence of organized distribution of antibiotics [19].
In our study, 21% of the decrease in the prevalence of active trachoma every 6 months (P = .001; 95% CI, 13%–29% decrease) could not be attributed to administration of antibiotics or to seasonal trends. This secular trend is dramatic and suggests that active trachoma might have disappeared rapidly in this area even if we had not instituted the antibiotic program. It is difficult to determine the exact cause of this decrease in the prevalence of trachoma. A lower prevalence of trachoma has been associated with a number of socioeconomic factors, including better hygiene, sanitation, and fly control and a cleaner water supply [8, 20–24]. No trachoma-control measures other than administration of antibiotics were specifically instituted in the 18 villages included in this study (e.g., no facial cleanliness campaign and no fly-control program). Apparently, the prevalence of trachoma may decrease because of general socioeconomic improvements independent of a trachoma-control program, and this decrease can occur fairly rapidly.
Trachoma is clearly disappearing in western Nepal. Antibiotic programs appear to be effective in the short term, even after accounting for chance, seasonal variation, and secular trends. However, we were not able to demonstrate that this treatment effect persisted as long as a year. Much of the long-term trend toward disappearance may, in fact, be due to a secular trend. As shown in this study, seasonal effects and secular trends can be quite significant, so investigators must be careful not to attribute all improvement to their trachoma-control program unless they use adequate controls. Trachoma-control programs may want to assess any local secular trends so that they do not overestimate the efficacy of their program and so that they may allocate the scarce resources available for the control of active trachoma to those geographic areas where the disease is not disappearing on its own.
Acknowledgments
We thank the following people for their invaluable help with the project: members of the staff at Geta Eye Hospital, including R. R. Bhatta, B. K. Jamuna, R. B. Chaudhary, G. B. Chaudhary, B. R. Chaudhary, B. S. Dhami, Batti Gurung, Manju Gurung, L. R. Panta, K. S. Khuna, and T. B. Deupa; Lisa Tapert, Bal Bahadur, Ganga Rana, and B. B. Thapa (the Helen Keller Institute); Ram Prasad Pokhrel and Diwash Rijal (Nepal Netra Jyoti Sangh); H. S. Bista (Norwegian Church Aid); Travis Porco (San Francisco Department of Public Health); Richard Stephens (the F. I. Proctor Foundation and the University of California, Berkeley, School of Public Health); and Stephanie Costanza (the F. I. Proctor Foundation).
References
Financial support: Edna McConnell Clark Foundation, Geta Eye Hospital, Helen Keller International, Nepal Netra Jyoti Sangh (National Society for Comprehensive Eye Care), Pfizer (which donated azithromycin [Zithromax]), National Institutes of Health (grant AI-01441), South Asia Research Fund, and F. I. Proctor Foundation.





Comments