Abstract
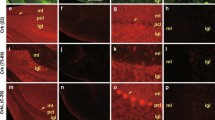
Two-photon microscopy was used to image dye-loaded filopodia of Purkinje cells in acute rat cerebellar slices. In the process of examining filopodia in Purkinje cells from a period of rapid dendritic growth (P10-21), we observed a small subset of filopodia which appeared to form connections between two dendrites of the same cell, usually between the tips of two adjacent dendrites or the tip of a dendrite and the shaft of another. There were fewer of these ‘filopodial bridges’ present at P18-21 than at an earlier stage in development (P10-12) and they were absent in mature Purkinje cells. Filopodial bridges do not appear to be an artifact of living brain slice preparation as they may also be seen by dye-loading Purkinje cells in slices prepared from perfusion-fixed brain. They have varied morphologies which are mostly similar to conventional, unattached filopodia. However, when measured over tens of minutes, filopodial bridges were observed to be less motile than conventional filopodia as indicated by a reduced index of expansion. While the functions of these novel structures are unknown it is attractive to speculate that they play an instructive role in Purkinje cell dendritic development
Similar content being viewed by others
References
Hausser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science. 2000;290:739–44.
Hausser M, Mel B. Dendrites: Bug or feature? Curr Opin Neurobiol. 2003;13:372–83.
Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3: 803–12.
Scott EK, Luo L. How do dendrites take their shape? Nat Neurosci. 2001;4:359–65.
McAllister AK. Cellular and molecular mechanisms of dendrite growth. Cereb Cortex. 2000;10:963–73.
Jan YN, Jan LY. Dendrites. Genes Dev. 2001;15:2627–41.
Jan YN, Jan LY. The control of dendrite development. Neuron. 2003;40:229–42.
Whitford KL, Dijkhuizen P, Polleux F, Ghosh A. Molecular control of cortical dendrite development. Annu Rev Neurosci. 2002;25:127–49.
Morest DK. The growth of dendrites in the mammalian brain. Z Anat Entwicklungsgesch. 1969;128:290–317.
Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004; 7:254–60.
Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–94.
Portera-Cailliau C, Pan DT, Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia. J Neurosci. 2003;23: 7129–42.
Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–7.
Wang H, Macagno ER. The establishment of peripheral sensory arbors in the leech: In vivo time-lapse studies reveal a highly dynamic process. J Neurosci. 1997;17:2408–19.
Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, et al. Long-term in vivo imaging of experiencedependent synaptic plasticity in adult cortex. Nature. 2002;420:788–94.
Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–6.
Vaughn JE. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse. 1989;3:255–85.
Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–11.
Petrak LJ, Harris KM, Kirov SA. Synaptogenesis on mature hippocampal dendrites occurs via filopodia and immature spines during blocked synaptic transmission. J Comp Neurol. 2005;484:183–90.
Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102.
Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–54.
Roelandse M, Welman A, Wagner U, Hagmann J, Matus A. Focal motility determines the geometry of dendritic spines. Neuroscience. 2003;121:39–49.
Napper RM, Harvey RJ. Number of parallel fiber synapses on an individual Purkinje cell in the cerebellum of the rat. J Comp Neurol. 1988;274:168–77.
Strata P, Rossi F. Plasticity of the olivocerebellar pathway. Trends Neurosci. 1998;21:407–13.
Jaeger D, De Schutter E, Bower JM. The role of synaptic and voltage-gated currents in the control of Purkinje cell spiking: a modeling study. J Neurosci. 1997;17:91–106.
Xu-Friedman MA, Harris KM, Regehr WG. Threedimensional comparison of ultrastructural characteristics at depressing and facilitating synapses onto cerebellar Purkinje cells. J Neurosci. 2001;21:6666–72.
Sotelo C. Cerebellar synaptogenesis: what we can learn from mutant mice. J Exp Biol. 1990;153:225–49.
Berry M, Bradley P. The growth of the dendritic trees of Purkinje cells in the cerebellum of the rat. Brain Res. 1976;112:1–35.
Del Cerro MP, Snider RS. Studies on the developing cerebellum. Ultrastructure of the growth cones. J Comp Neurol. 1968;133:341–62.
Dunaevsky A, Tashiro A, Majewska A, Mason C, Yuste R. Developmental regulation of spine motility in the mammalian central nervous system. Proc Natl Acad Sci USA. 1999;96:13438–43.
Harris KM, Kater SB. Dendritic spines: Cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–71.
Wallace W, Bear MF. A morphological correlate of synaptic scaling in visual cortex. J Neurosci. 2004;24:6928–38.
Vecellio M, Schwaller B, Meyer M, Hunziker W, Celio MR. Alterations in Purkinje cell spines of calbindin D-28 k and parvalbumin knock-out mice. Eur J Neurosci. 2000;12: 945–54.
Konur S, Rabinowitz D, Fenstermaker VL, Yuste R. Systematic regulation of spine sizes and densities in pyramidal neurons. J Neurobiol. 2003;56:95–112.
Wallace W, Schaefer LH, Swedlow JR. A working person’s guide to deconvolution in light microscopy. Biotechniques. 2001;31:1076–8, 1080, 1082 passim.
Altman J, Bayer SA. Development of the cerebellar system: In relation to its evolution, structure, and functions. Boca Raton: CRC Press; 1997.
Kirov SA, Petrak LJ, Fiala JC, Harris KM. Dendritic spines disappear with chilling but proliferate excessively upon rewarming of mature hippocampus. Neuroscience. 2004;127: 69–80.
Kirov SA, Sorra KE, Harris KM. Slices have more synapses than perfusion-fixed hippocampus from both young and mature rats. J Neurosci. 1999;19:2876–86.
Fiala JC, Kirov SA, Feinberg MD, Petrak LJ, George P, Goddard CA, et al. Timing of neuronal and glial ultrastructure disruption during brain slice preparation and recovery in vitro. J Comp Neurol. 2003;465:90–103.
Mori M, Matsushima T. Post-hatch development of dendritic arborization in cerebellar Purkinje neurons of quail chicks: A morphometric study. Neurosci Lett. 2002;329:73–6.
Takacs J, Hamori J. Developmental dynamics of Purkinje cells and dendritic spines in rat cerebellar cortex. J Neurosci Res. 1994;38:515–30.
Lendvai B, Stern EA, Chen B, Svoboda K. Experiencedependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–81.
Dunaevsky A, Blazeski R, Yuste R, Mason C. Spine motility with synaptic contact. Nat Neurosci. 2001;4:685–6.
Robinson DN, Cooley L. Stable intercellular bridges in development: the cytoskeleton lining the tunnel. Trends Cell Biol. 1996;6:474–9.
Witkin JW, O’Sullivan H, Silverman AJ. Novel associations among gonadotropin-releasing hormone neurons. Endocrinology. 1995;136:4323–30.
Yamada K, Fukaya M, Shibata T, Kurihara H, Tanaka K, Inoue Y, et al. Dynamic transformation of Bergmann glial fibers proceeds in correlation with dendritic outgrowth and synapse formation of cerebellar Purkinje cells. J Comp Neurol. 2000;418:106–20.
MacNeil MA, Masland RH. Extreme diversity among amacrine cells: Implications for function. Neuron. 1998;20: 971–82.
Wassle H, Peichl L, Boycott BB. Dendritic territories of cat retinal ganglion cells. Nature. 1981;292:344–5.
Blackshaw SE, Nicholls JG, Parnas I. Expanded receptive fields of cutaneous mechanoreceptor cells after single neurone deletion in leech central nervous system. J Physiol. 1982; 326:261–8.
Emoto K, He Y, Ye B, Grueber WB, Adler PN, Jan LY, et al. Control of dendritic branching and tiling by the Tricorneredkinase/Furry signaling pathway in Drosophila sensory neurons. Cell. 2004;119:245–56.
Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–78.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sdrulla, A.D., Linden, D.J. Dynamic imaging of cerebellar Purkinje cells reveals a population of filopodia which cross-link dendrites during early postnatal development. Cerebellum 5, 105–115 (2006). https://doi.org/10.1080/14734220600620908
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1080/14734220600620908