Abstract
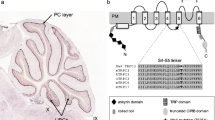
Mice with theweaver mutation exhibit an uneven weave to their gait, ataxia, mild locomotor hyperactivity and, occasionally, tonic-clonic seizures. A single amino acid mutation in a G-protein coupled, inwardly rectifying K+ channel, GIRK2, gives rise to the symptoms seen in theweaver mice. Two areas of the brain are primarily affected. Cerebellar granule cell neurons die soon after birth and dopaminergic neurons are severely depleted in the substantia nigra. In this article we review recent studies of wild-type and mutant GIRK channels found in native cells or introduced into expression systems. We also review two models that explain some of the details leading to the neuronal cell death observed inweaver mice.
Similar content being viewed by others
References
Rezai Z, Yoon CH. Abnormal rate of granule cell migration in the cerebellum of weaver mutant mice. Dev Biol 1972; 29: 17–26.
Rakic P, Sidman RL. Weaver mutant mouse cerebellum: defective neuronal migration secondary to abnormality of Bergmann glia. Proc Natl Acad Sci USA 1973; 70: 240–244.
Sidman RL. Development of interneuronal connections in brains of mutant mice. In: Carlson FD, editor. Physiological and biochemical aspects of nervous integration. Englewood Cliffs: Prentice-Hall Inc., 1968: 163–193.
Schmidt MJ, Sawyer BD, Perry KW, Fuller RW, Foreman MM, Ghetti B. Dopamine deficiency in the weaver mutant mouse. J Neurosci 1982; 2: 376–380.
Vogelweid CM, Verina T, Norton J, Harruff R, Ghetti B. Hypospermatogenesis is the cause of infertility in the male weaver mutant mouse. J Neurogenet 1993; 9: 89–104.
Eisenberg B, Messer A. Tonic/clonic seizures in a mouse mutant carrying the weaver gene. Neurosci Lett 1989; 96: 168–172.
Goldowitz D. The weaver granuloprival phenotype is due to intrinsic action of the mutant locus in granule cells: evidence from homozygous weaver chimeras. Neuron 1989; 2: 1565–1575.
Hatten ME, Liem RK, Mason CA. Defects in specific associations between astroglia and neurons occur in microcultures of weaver mouse cerebellar cells. J Neurosci 1984; 4: 1163–1172.
Hatten ME, Liem RK, Mason CA. Weaver mouse cerebellar granule neurons fail to migrate on wild-type astroglial processes in vitro. J Neurosci 1986; 6: 2676–2683.
Gao WQ, Hatten ME. Neuronal differentiation rescued by implantation of Weaver granule cell precursors into wild-type cerebellar cortex. Science 1993; 260: 367–369.
Sidman RL. Cell surface properties and the expression of inherited brain diseases in mice. In: Bolis L, editor. Membranes and disease. New York: Raven Press, 1976: 379–386.
Mullen RJ, Eicher EM, Sidman RL. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc Natl Acad Sci USA 1976; 73: 208–212.
Maricich SM, Soha J, Trenkner E, Herrup K. Failed cell migration and death of Purkinje cells and deep nuclear neurons in the weaver cerebellum. J Neurosci 1997; 17: 3675–3683.
Roffler-Tarlov S, Martin B, Graybiel AM, Kauer JS. Cell death in the midbrain of the murine mutation weaver. J Neurosci 1996; 16: 1819–1826.
Triarhou LC, Norton J, Ghetti B. Mesencephalic dopamine cell deficit involves areas A8, A9 and A10 in weaver mutant mice. Exp Brain Res 1988; 70: 256–265.
Sekiguchi M, Nowakowski RS, Nagato Y, Tanaka O, Guo H, Madoka M, Abe H. Morphological abnormalities in the hippocampus of the weaver mutant mouse. Brain Res 1995; 696: 262–267.
Verina T, Tang X, Fitzpatrick L, Norton J, Vogelweid C, Ghetti B. Degeneration of Sertoli and spermatogenic cells in homozygous and heterozygous weaver mice. J. Neurogenet 1995; 9: 251–265.
Harrison SM, Roffler-Tarlov SK. Cell death during development of testis and cerebellum in the mutant mouse weaver. Dev Biol 1998; 195: 174–186.
Patil N, Cox DR, Bhat D, Faham M, Myers RM, Peterson AS. A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nat Genet 1995; 11: 126–129.
MacKinnon R, Yellen G. Mutations affecting TEA blockade and ion permeation in voltage-activated K+ channels. Science 1990; 250: 276–279.
Kofuji P, Hofer M, Millen KJ, Millonig JH, Davidson N, Lester HA, Hatten ME. Functional analysis of the weaver mutant GIRK2 K+ channel and rescue of weaver granule cells. Neuron 1996; 16: 941–952.
Silverman SK, Kofuji P, Dougherty DA, Davidson N, Lester HA. A regenerative link in the ionic fluxes through the weaver potassium channel underlies the pathophysiology of the mutation. Proc Natl Acad Sci USA 1996; 93: 15429–15434.
Navarro B, Kennedy ME, Velimirovic B, Bhat D, Peterson AS, Clapham DE. Nonselective and G betagamma-insensitive weaver K+ channels. Science 1996; 272: 1950–1953.
Slesinger PA, Patil N, Liao YJ, Jan YN, Jan LY, Cox DR. Functional effects of the mouse weaver mutation on G protein-gated inwardly rectifying K+ channels. Neuron 1996; 16: 321–331.
Tong Y, Wei J, Zhang S, Strong JA, Dlouhy SR, Hodes ME, Ghetti B, Yu L. The weaver mutation changes the ion selectivity of the affected inwardly rectifying potassium channel GIRK2. FEBS Lett 1996; 390: 63–68.
Hille B. Ion channels of excitable membranes. Sunderland: Sinauer, 1992.
Jan LY, Jan YN. Voltage-gated and inwardly rectifying potassium channels. J Physiol 1997; 505: 267–282.
Mark MD, Herlitze S. G-protein mediated gating of inward-rectifier K+ channels. Eur J Biochem 2000; 267: 5830–5836.
Sui JL, Petit-Jacques J, Logothetis DE. Activation of the atrial KACh channel by the betagamma subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc Natl Acad Sci USA 1998; 95: 1307–1312.
Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature 1998; 391: 803–806.
Kobrinsky E, Mirshahi T, Zhang H, Jin T, Logothetis DE. Receptor-mediated hydrolysis of plasma membrane messenger PIP2 leads to K+-current desensitization. Nat Cell Biol 2000; 2: 507–514.
Sadja R, Smadja K, Alagem N, Reuveny E. Coupling Gbetagamma-dependent activation to channel opening via pore elements in inwardly rectifying potassium channels. Neuron 2001; 29: 669–680.
Kobayashi T, Ikeda K, Kojima H, Niki H, Yano R, Yoshioka T, Kumanishi T. Ethanol opens G-protein-activated inwardly rectifying K+ channels. Nat Neurosci 1999; 2: 1091–1097.
Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, Harris RA. G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci 1999; 2: 1084–1090.
Zhou W, Arrabit C, Choe S, Slesinger PA. Mechanism underlying bupivacaine inhibition of G protein-gated inwardly rectifying K+ channels. Proc Natl Acad Sci USA 2001; 98: 6482–6487.
Jan LY, Jan YN. Heartfelt crosstalk: desensitization of the GIRK current. Nat Cell Biol 2000; 2: E165–167.
Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature 1993; 362: 31–38.
Kubo Y, Reuveny E, Slesinger PA, Jan YN, Jan LY. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature 1993; 364: 802–806.
Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature 1993; 362: 127–133.
Lesage F, Duprat F, Fink M, Guillemare E, Coppola T, Lazdunski M, Hugnot JP. Cloning provides evidence for a family of inward rectifier and G-protein coupled K+ channels in the brain. FEBS Lett 1994; 353: 37–42.
Kofuji P, Davidson N, Lester HA. Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by G beta gamma subunits and function as heteromultimers. Proc Natl Acad Sci USA 1995; 92: 6542–6546.
Lesage F, Guillemare E, Fink M, Duprat F, Heurteaux C, Fosset M, Romey G, Barhanin J, Lazdunski M. Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. J Biol Chem 1995; 270: 28660–28667.
Andrade R, Malenka RC, Nicoll RA. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science 1986; 234: 1261–1265.
North RA, Williams JT, Surprenant A, Christie MJ. Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci USA 1987; 84: 5487–5491.
Williams JT, Colmers WF, Pan ZZ. Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. J Neurosci 1988; 8: 3499–3506.
Kovoor A, Henry DJ, Chavkin C. Agonist-induced desensitization of the mu opioid receptor-coupled potassium channel (GIRK1). J Biol Chem 1995; 270: 589–595.
Penington NJ, Kelly JS, Fox AP. Whole-cell recordings of inwardly rectifying K+ currents activated by 5-HT1A receptors on dorsal raphe neurones of the adult rat. J Physiol 1993; 469: 387–405.
Penington NJ, Kelly JS, Fox AP. Action potential waveforms reveal simultaneous changes in ICa and IK produced by 5-HT in rat dorsal raphe neurons. Proc R Soc Lond B Biol Sci 1992; 248: 171–179.
Wickman KD, Iniguez-Lluhl JA, Davenport PA, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE. Recombinant G-protein beta gamma-subunits activate the muscarinic-gated atrial potassium channel. Nature 1994; 368: 255–257.
Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature 1996; 380: 255–258.
Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature 1996; 380: 258–262.
Nakajima Y, Nakajima S, Kozasa T. Activation of G protein-coupled inward rectifier K+ channels in brain neurons requires association of G protein beta gamma subunits with cell membrane. FEBS Lett 1996; 390: 217–220.
Kobayashi T, Ikeda K, Ichikawa T, Abe S, Togashi S, Kumanishi T. Molecular cloning of a mouse G-protein-activated K+ channel (mGIRK1) and distinct distributions of three GIRK (GIRK1, 2 and 3) mRNAs in mouse brain. Biochem Biophys Res Commun 1995; 208: 1166–1173.
Karschin C, Dissmann E, Stuhmer W, Karschin A. IRK(1–3) and GIRK(1–4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci 1996; 16: 3559–3570.
Hess EJ. Identification of the weaver mouse mutation: the end of the beginning. Neuron 1996; 16: 1073–1076.
Chen SC, Ehrhard P, Goldowitz D, Smeyne RJ. Developmental expression of the GIRK family of inward rectifying potassium channels: implications for abnormalities in the weaver mutant mouse. Brain Res 1997; 778: 251–264.
Wei J, Dlouhy SR, Bayer S, Piva R, Verina T, Wang Y, Feng Y, Dupree B, Hodes ME, Ghetti B. In situ hybridization analysis of Girk2 expression in the developing central nervous system in normal and weaver mice. J Neuropathol Exp Neurol 1997; 56: 762–771.
Triarhou LC, Low WC, Ghetti B. Transplantation of ventral mesencephalic anlagen to hosts with genetic nigrostriatal dopamine deficiency. Proc Natl Acad Sci USA 1986; 83: 8789–8793.
Schein JC, Hunter DD, Roffler-Tarlov S. Girk2 expression in the ventral midbrain, cerebellum, and olfactory bulb and its relationship to the murine mutation weaver. Dev Biol 1998; 204: 432–450.
Liao YJ, Jan YN, Jan LY. Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J Neurosci 1996; 16: 7137–7150.
Signorini S, Liao YJ, Duncan SA, Jan LY, Stoffel M. Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci USA 1997; 94: 923–927.
Duprat F, Lesage F, Guillemare E, Fink M, Hugnot JP, Bigay J, Lazdunski M, Romey G, Barhanin J. Heterologous multimeric assembly is essential for K+ channel activity of neuronal and cardiac G-protein-activated inward rectifiers. Biochem Biophys Res Commun 1995; 212: 657–663.
Slesinger PA, Stoffel M, Jan YN, Jan LY. Defective gamma-aminobutyric acid type B receptor-activated inwardly rectifying K+ currents in cerebellar granule cells isolated from weaver and Girk2 null mutant mice. Proc Natl Acad Sci USA 1997; 94: 12210–12217.
Hou P, Yan S, Tang W, Nelson DJ. The inwardly rectifying K(+) channel subunit GIRK1 rescues the GIRK2 weaver phenotype. J Neurosci 1999; 19: 8327–8336.
Silverman SK, Lester HA, Dougherty DA. Subunit stoichiometry of a heteromultimeric G protein-coupled inward-rectifier K+ channel. J Biol Chem 1996; 271: 30524–30528.
Corey S, Krapivinsky G, Krapivinsky L, Clapham DE. Number and stoichiometry of subunits in the native atrial G-protein-gated K+ channel, IKACh. J Biol Chem 1998; 273: 5271–5278.
Mjaatvedt AE, Cabin DE, Cole SE, Long LJ, Breitwieser GE, Reeves RH. Assessment of a mutation in the H5 domain of GIRK2 as a candidate for the weaver mutation. Genome Res 1995; 5: 453–463.
Surmeier DJ, Mermelstein PG, Goldowitz D. The weaver mutation of GIRK2 results in a loss of inwardly rectifying K+ current in cerebellar granule cells. Proc Natl Acad Sci USA 1996; 93: 11191–11195.
Lauritzen I, De Weille J, Adelbrecht C, Lesage F, Murer G, Raisman-Vozari R, Lazdunski M. Comparative expression of the inward rectifier K+ channel GIRK2 in the cerebellum of normal and weaver mutant nice. Brain Res 1997; 753: 8–17.
Rossi P, De Filippi G, Armano S, Taglietti V, D'Angelo E. The weaver mutation causes a loss of inward rectifier current regulation in premigratory granule cells of the mouse cerebellum. J Neurosci 1998; 18: 3537–3547.
Choi DW. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci 1995; 18: 58–60.
Sui JL, Chan K, Langan MN, Vivaudou M, Logothetis DE. G protein gated potassium channels. Adv Second Messenger Phosphoprotein Res 1999; 33: 179–201.
Womack M, Thompson K, Fanselow E, Augustine GJ, Peterson A. Elevated intracellular calcium levels in cerebellar granule neurons of weaver mice. Neuroreport 1998; 9: 3391–3395.
Fox AP, Dlouhy S, Ghetti B, Hurley JH, Nucifora PG, Nelson DJ, Won L, Heller A. Altered responses to potassium in cerebellar neurons from weaver heterozygote mice. Exp Brain Res 1998; 123: 298–306
Harkins AB, Dlouhy S, Ghetti B, Cahill AL, Won L, Heller B, Heller A, Fox AP. Evidence of elevated intracellular calcium levels in weaver homozygote mice. J Physiol 2000; 524 Pt 2: 447–455.
Murtomaki S, Trenkner E, Wright JM, Saksela O, Liesi P. Increased proteolytic activity of the granule neurons may contribute to neuronal death in the weaver mouse cerebellum. Dev Biol 1995; 168: 635–648.
Komuro H, Rakic P. Distinct modes of neuronal migration in different domains of developing cerebellar cortex. J Neurosci 1998; 18: 1478–1490.
Liesi P, Wright JM. Weaver granule neurons are rescued by calcium channel antagonists and antibodies against a neurite out-growth domain of the B2 chain of laminin. J Cell Biol 1996; 134: 477–486.
Tucker SJ, Pessia M, Moorhouse AJ, Gribble F, Ashcroft FM, Maylie J, Adelman JP. Heteromeric channel formation and Ca(2+)-free media reduce the toxic effect of the weaver Kir 3.2 allele. FEBS Lett 1996; 390: 253–257.
Liesi P, Wright JM, Krauthamer V. BAPTA-AM and ethanol protect cerebellar granule neurons from the destructive effect of the weaver gene. J Neurosci Res 1997; 48: 571–579.
Slesinger PA. Ion selectivity filter regulates local anesthetic inhibition of G-protein-gated inwardly rectifying K+ channels. Biophys J 2001; 80: 707–718.
Liesi P, Stewart RR, Wright JM. Involvement of GIRK2 in postnatal development of the weaver cerebellum. J Neurosci Res 2000; 60: 164–173.
Forrest D, Yuzaki M, Soares HD, Ng L, Luk DC, Sheng M, Stewart CL, Morgan JI, Connor JA, Curran T. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron 1994; 13: 325–338.
Rossi DJ, Slater NT. The developmental onset of NMDA receptor-channel activity during neuronal migration. Neuropharmacology 1993; 32: 1239–1248.
Farrant M, Feldmeyer D, Takahashi T, Cull-Candy SG. NMDA-receptor channel diversity in the developing cerebellum. Nature 1994; 368: 335–339.
Jensen P, Surmeier DJ, Goldowitz D. Rescue of cerebellar granule cells from death in weaver NR1 double mutants. J Neurosci 1999; 19: 7991–7998.
Tymianski M, Spigelman I, Zhang L, Carlen PL, Tator CH, Charlton MP, Wallace MC. Mechanism of action and persistence of neuroprotection by cell-permeant Ca2+ chelators. J Cereb Blood Flow Metab 1994; 14: 911–923.
McConkey DJ, Orrenius S. The role of calcium in the regulation of apoptosis. Biochem Biophys Res Commun 1997; 239: 357–366.
Gao WQ, Liu XL, Hatten ME. The weaver gene encodes a nonautonomous signal for CNS neuronal differentiation. Cell 1992; 68: 841–854.
Won L, Ghetti B, Heller B, Heller A. In vitro evidence that the reduction in mesencephalic dopaminergic neurons in the weaver heterozygote is not due to a failure in target cell interaction. Exp Brain Res 1997; 115: 174–179.
Liss B, Neu A, Roeper J. The weaver mouse gain-of-function phenotype of dopaminergic midbrain neurons is determined by coactivation of wvGirk2 and K-ATP channels. J Neurosci 1999; 19: 8839–8848.
Radnikow G, Titz S, Mades S, Baurle J, Misgeld U. Gamma-aminobutyric acid(B) autoreceptors in substantia nigra and neostriatum of the weaver mutant mouse. Neurosci Lett 2001; 299: 81–84.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harkins, A.B., Fox, A.P. Cell death inweaver mouse cerebellum. Cerebellum 1, 201–206 (2002). https://doi.org/10.1080/14734220260418420
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1080/14734220260418420