-
PDF
- Split View
-
Views
-
Cite
Cite
Ga-Hyun Joe, Midori Andoh, Mikako Nomura, Hitoshi Iwaya, Jae-Sung Lee, Hidehisa Shimizu, Youhei Tsuji, Hideaki Maseda, Hitoshi Miyazaki, Hiroshi Hara, Satoshi Ishizuka, Acyl-homoserine lactones suppresses IEC-6 cell proliferation and increase permeability of isolated rat colon, Bioscience, Biotechnology, and Biochemistry, Volume 78, Issue 3, 4 March 2014, Pages 462–465, https://doi.org/10.1080/09168451.2014.882748
Close - Share Icon Share
Abstract
We investigated to determine whether a variety of acyl-homoserine lactones (AHLs) influences epithelial cell proliferation and mucosal permeability. 3-Oxo-C12-homoserine lactone (HSL) and 3-oxo-C14-HSL significantly suppressed IEC-6 cell proliferation. A significant increase in mucosal permeability was observed in isolated rat colon tissue exposed to C12-HSL, 3-oxo-C12-HSL, and 3-oxo-C14-HSL. These data indicate that AHLs suppress epithelial proliferation and disrupt barrier function in intestinal mucosa.
Acyl-homoserine lactones (AHLs) play an important role in the transduction of signals among bacteria in a process known as quorum sensing.1) Quorum sensing is a form of cell-to-cell communication that bacteria use to regulate gene expression in response to changes in population density.2) In quorum sensing, an autoinducer is a signaling molecule that enables bacteria to sense their density and control the expression of virulence factors. AHL is an autoinducer produced by some Gram-negative bacteria such as Pseudomonas aeruginosa3–5) that regulates various genes involved in virulence and biofilm formation.6) In recent years, 3-oxo-C12-homoserine lactone (HSL) has been reported to affect the immune response of host cells.7) There are few reports on the action of HSLs other than 3-oxo-C12-HSL, especially against host cells. In this experiment, we investigated to determine whether various types of AHL influence cell proliferation at different concentrations in the IEC-6 epithelial cell line derived from the rat small intestine. We also investigated the effects of AHLs on epithelial permeability with ex vivo tissue samples isolated from the rat colon.
The N-acyl-l-homoserine lactones (HSL; C8-HSL, C10-HSL, C12-HSL, 3-oxo-C8-HSL, 3-oxo-C10-HSL, 3-oxo-C12-HSL, and 3-oxo-C14-HSL) used in this study were synthesized chemically (Fig. 1(A)). The IEC-6 cell line was obtained (ATCCCRL 1592) from the American Type Culture Collection (Manassas, VA). This cell line is used as a model of undifferentiated intestinal epithelial cells and would not have tight junctions.8) No mycoplasma was detected in the cultures. The cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, 12100-046; Gibco®, Invitrogen Co., Carlsbad, CA) supplemented with 5% FBS, 0.1 of U/mL insulin, 35 mg/L of penicillin G potassium, and 100 mg/L of streptomycin sulfate and incubated at 37 °C in a humidified atmosphere of 95% air and 5% CO2.9) They were seeded at 1,500 cells/well on 96-well plates and incubated in DMEM for 24 h. Then, they were exposed to DMEM-containing AHL at the indicated concentrations for 48 h. AHL was dissolved in dimethylsulfoxide (DMSO), and the final concentration of DMSO was 0.5% in the medium. The cells were measured by Cell Counting Kit-8 Assay (Dojindo Laboratories, Kumamoto, Japan). For the cell cycle analysis, the cells were seeded at 1.5 × 106 cells/dish (10 cm) and cultured for 24 h. Then, the cells were exposed to DMEM with or without C8-HSL, C10-HSL, C12-HSL, 3-oxo-C8-HSL, 3-oxo-C10-HSL, 3-oxo-C12-HSL, or 3-oxo-C14-HSL at 100 μM for 18 h. The harvested cells were fixed with 70% ethanol and stained with propidium iodide. The proportion of the cells in sub-G1 phase was determined by FACSCalibur (Becton, Dickson and Company, Franklin Lakes, NJ).10)
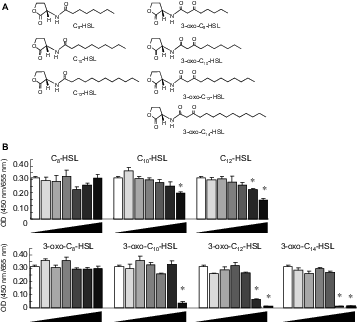
Molecular structure of the AHLs used in this study (A) and IEC-6 cell proliferation in response to supplementation with AHL (B).
Note: Cells were exposed to various concentrations (0, 10−9, 10−8, 10−7, 10−6, 10−5, and 10−4 m) of AHLs (C8-HSL, C10-HSL, C12-HSL, 3-oxo-C8-HSL, 3-oxo-C10-HSL, 3-oxo-C12-HSL, and 3-oxo-C14-HSL). The highest concentration appears to the right in each panel. Values are arbitrary units and are expressed as averages with SEM (n = 4).
*Significant difference from control (p < 0.05).
WKAH/HkmSlc male rats (5 weeks old; Japan SLC, Hamamatsu, Japan) were housed individually in stainless steel cages with wire-mesh bottoms. The cages were in a room with a controlled temperature (22 ± 2 °C), relative humidity (40–60%), and lighting (lights on 08:00–20:00) throughout the day. The rats were fed a diet based on the AIN-93G formula.11) All of the experiments were approved by the Hokkaido University Animal Committee, and the rats were maintained in accordance with the guidelines of Hokkaido University for the care and use of laboratory animals.
The Ussing chamber provides a physiological system to measure the transport of ions, nutrients, and drugs across various epithelial tissues.12) Colon tissues isolated from the rats were mounted in an Ussing chamber as flat sheets on a segment holder, with a surface area of 1 cm2. Hank’s balanced salt solution (HBSS, pH 7.4)13) was introduced on both sides of the mucosa and incubated for 10 min at 37 °C. After the transepithelial resistance was measured, the buffer on the mucosal side was replaced with 0.1% DMSO in HBSS containing Lucifer yellow (LY; final concentration, 100 μM) with AHL. The concentration of AHL was 0, 1, or 10 μM. The buffer on the serosal side was collected after 60 min, and the LY concentration was measured. Statistical analyses were carried out with JMP software ver 10.0 (SAS Institute Inc., Cary, NC). All of the results were expresses as the means with SEMs. Significant differences as compared to control were determined by Dunnett’s test. The Tukey–Kramer test was used to evaluate significant differences in values among the treatments. A probability of less than 0.05 was considered to represent a significant difference.
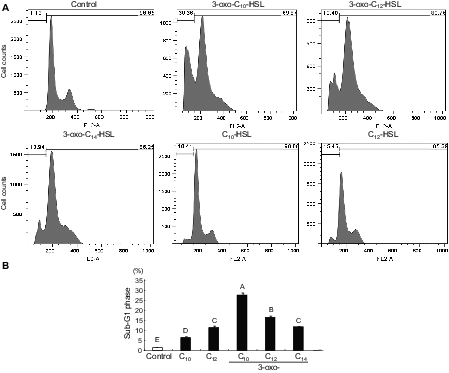
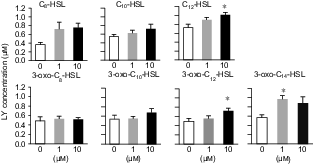
There was a massive reduction in IEC-6 cell proliferation in the cells exposed to 3-oxo-C14-HSL even at 10 μM (Fig. 1(B)). Similar effects were caused by supplementation with 3-oxo-C12-HSL at 10 μM and with 3-oxo-C10-HSL at 100 μM. The influence was relatively weak for the C10- and C12-HSLs as compared to the oxo form. The longer acyl-chain with the 3-oxo form appeared to have a strongly suppressive effect on cell proliferation. No significant effect was found in the cells supplemented with C8-HSL in this concentration range, regardless of the presence of the oxo form. In flow cytometry analysis, the proportion of IEC-6 cells in sub-G1 phase in response to AHLs increased significantly, as compared to control (Fig. 2). Compared with the acyl types of C10- and C12-HSLs, the oxo types increased the proportion of sub-G1 phase cells in the cultures. The oxo-HSLs with longer acyl chains reduced the proportion of sub-G1 cells, while the acyl-HSLs with longer acyl chains increased the sub-G1 population. In addition, the distribution of the cells in the S and G2/M phases was not clear in the oxo-HSL-supplemented IEC-6 cells. In the ex vivo mucosal permeability study using isolated rat colonic mucosa (Fig. 3), 3-oxo-C14 HSL significantly promoted LY permeability at 1 μM. HSLs with longer acyl chains, such as C12-HSL and 3-oxo-C12-HSL, significantly increased LY permeability at 10 μM as well.
Proportion of the sub-G1 phase in IEC-6 cells subjected to AHL treatment.
Note: Cells were exposed to AHLs (control, C10-HSL, C12-HSL, 3-oxo-C10-HSL, 3-oxo-C12-HSL, or 3-oxo-C14-HSL) at 100 μM for 18 h. Representative histograms of propidium iodide incorporation in the cells in response to each of the AHL (A). Percentages of cells in the sub-G1 phase in response to each of the AHL (B). Values are expressed as averages with SEM (n = 4). Values with different letters are significantly different (p < 0.05).
Effects of AHLs on mucosal permeability of the isolated rat colon.
Note: Colonic mucosa was isolated from rats and mounted in an Ussing chamber. Lucifer yellow (100 μM) and AHLs were added to the mucosal chamber and this was incubated for 60 min. The LY concentration in the serosal chamber was measured as permeability marker. Values are arbitrary units and are expressed as averages with SEM (n = 4).
*Significant difference from control (p < 0.05).
This is the first study showing that certain AHLs suppressed epithelial cell proliferation in the IEC-6 cell line and disrupted barrier function in isolated rat colonic mucosa. 3-Oxo-C12-HSL (50 μM) induced a significant loss of viability in neutrophils and two monocyte cell lines (U-937 and P388D1 cells), but not in two epithelial cell lines (CCL-185 and HEp-2 cells).14) Other studies have found that 3-oxo-C12-HSL does not induce cell death in Caco-2 cells, even at 200 μM.15) In that experiment, they used differentiated Caco-2 cells and did not evaluate the cell proliferation in response to 3-oxo-C12 HSL. Differentiated Caco-2 cells might be resistant to 3-oxo-C12 HSL, and it is possible that 3-oxo-C12 HSL reduces cell viability, as it increases apoptosis in proliferating Caco-2 cells.
To date, no study has demonstrated that 3-oxo-C12-HSL induces epithelial cell death in this range of concentrations. Proliferation tests might be more sensitive for detecting cell viability than viability tests.
In this study, we investigated cell proliferation in the IEC-6 epithelial cell line, derived from rat small intestine. We found that 3-oxo-C12-HSL at 10 μM caused a significant reduction in cell proliferation (Fig. 1(B)). Other HSLs, such as C12 and 3-oxo-C14 HSLs, also suppressed IEC-6 cell proliferation at the same concentration. We found that C10 and 3-oxo-C10-HSLs decreased proliferation at 100 μM. These results suggest that a variety of AHLs, not just 3-oxo-C12-HSL, can suppress proliferation in epithelial cells. We also conducted a cell-cycle analysis of IEC-6 cells in response to AHLs (Fig. 2) and found that the oxo types of HSLs enabled the induction of apoptosis in IEC-6 cells at 100 μM. In contrast, the acyl types of HSLs induced cell-cycle arrest at the same concentration. Differences in the acyl chain length and the existence of the 3-oxo form appear to regulate the cell cycle by different mechanisms. 3-Oxo-C12-HSL inhibits PPARγ activity even in the presence of a potent agonist, rosiglitazone, in NIH 3T3 cells.16) In contrast, C4-HSL did not suppress such activation. These results suggest that inhibitory effect of HSL on PPARγ activity depends on differences in the acyl chain length and/or the presence of the 3-oxo residue.
3-Oxo-C12-HSL has been reported to reduce transepithelial resistance and to increase the permeability of FITC-dextran in Caco-2 monolayers at 200 μM.17) 3-Oxo-C12-HSL can disrupt adherens junctions in Caco-2 cells, as evidenced by reductions in the expression and distribution of E-cadherin and β-catenin. 3-Oxo-C12-HSL causes modulation of junction proteins as well as alterations in the phosphorylation state of E-cadherin, β-catenin, and occludin, leading to the reassembly of junction protein complexes,18) but the concentration of 3-oxo-C12-HSL in that study was extremely high as compared to that in physiological events, even in pathologic bacteria. There might be another mechanism in a physiological concentration range.
A large number of bacteria are present in the large intestine rather than the small intestine. Assuming that the host ingests antibiotics, a massive alteration in microbiota is to be expected in the large intestine. Some drug-resistant bacteria might remain after such treatment. It is possible that the host sufferers the influence of AHL released by the drug-resistant bacteria. In the present study, we investigated the influence of a variety of AHLs on epithelial permeability in a relatively physiological system by means of ex vivo tissue samples isolated from the rat colon. Because colonic permeability increased due to supplementation of HSLs with long acyl chains (Fig. 3), we conclude that these HSLs are involved in the disruption of gastrointestinal barrier function.
In the literature, effects of HSLs on host cells have been reported at concentrations ranging from 50 to 100 μM regardless of cell type.14,17) This concentration range is higher than in the situation in which AHLs act as autoinducers in bacteria. In the present study, we found that AHLs influence host cells and tissues as bacterial autoinducers at physiological concentrations. In conclusion, our data demonstrate that 3-oxo-HSLs with longer acyl chains specifically induced apoptosis in an epithelial cell line, resulting in suppression of cell proliferation and increased permeability in an ex vivo sample of rat colonic mucosa.
Footnotes
Abbreviations: AHL, acyl-homoserine lactone; HSL, homoserine lactone; DMEM, Dulbecco’s modified eagle medium; DMSO, dimethylsulfoxide; HBSS, Hank’s balanced salt solution; LY, Lucifer yellow.
Acknowledgment
We thank the Regional Innovation Strategy Support Program of the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of the Japanese Government for financial support for S.I.