Summary
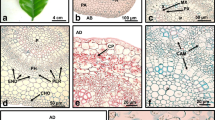
Leaf structure along the successive stages of Early French artichoke Cynara scolymus L. micropropagation was characterized using light and transmission electron microscopy. The mesophyll presents disorganized spongy and palisade parenchyma with large intercellular spaces and a few small chloroplasts in the leaves of plants cultured in vitro. In addition, both epidermal surfaces of such leaves invariably show a cell wall of the same thickness with a very thin cuticle and open stomata. In the root differentiation stage in vitro, structural changes take place in the leaves that are favorable for survival in the acclimatization stage: conspicuous cuticle, greater cell wall thickness, functional stomata, better mesophyll organization, developed vascular bundles, and the presence of sclerenchymatous tissue are observed. These features found in later in vitro stages are maintained in the following ex vitro stages, some becoming more evident. Our results demonstrate that the structural changes required to ensure appropriate acclimatization of micropropagated artichoke plants begin at the root differentiation stage, which can reduce in vivo acclimatization time and achieve greater survival of transferred plants.
Similar content being viewed by others
References
Apóstolo, N. M.; Brutti, C. B.; Ferrarotti, S. A.; Llorente, B. E.; Krymkiewicz, N. Stimulation of root development with cyclodextrins on jojoba shoots in vitro. In Vitro Cell. Dev. Biol. Plant 37:404–418; 2001.
Apóstolo, N. M.; Llorente, B. E. Anatomy of normal and hyperhydric leaves and shoots in vitro grown Simmondsia chinensis (Link) Schn. In Vitro Cell. Dev. Biol. Plant 36:243–249; 2000.
Brutti, C. B.; Apóstolo, N. M.; Ferrarotti, S. A.; Llorente, B. E.; Krymkiewicz, N. Micropropagation of Cynara scolymus L. employing cyclodextrins to promote rhizogenesis. Sci. Hort. 83:1–10; 2000.
Brutti, C. B.; Rubio, E.; Llorente, B. E.; Apóstolo, N. M. Artichoke leaf morphology and surface features in different micropropagation stages. Biol. Plant. 45:197–204; 2002.
Donnelly, D. J.; Skelton, F. E.; Daubeny, H. A. External leaf features of tissue-cultured Silvan blackberry. HortScience 21:306–308; 1986.
Donnelly, D. J.; Tisdall, L. Acclimatization strategies for micropropagated plants. In: Ahuja M. R., ed. Micropropagation of woody plants. Dordrecht: Kluwer Academic Publishers; 1993:153–156.
Freeman, B.; Albrigo, L. G.; Briggs, B. H. Cuticular waxes of developing leaves and fruit of blueberry Vaccimium ashei Reade cv. Bluegem. J. Am. Soc. Hort. Sci. 194:398–403; 1979.
Gamborg, O. L.; Miller, R.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50:151–158; 1968.
Heller, R. Recherches sur la nutrition minerales des tissues végétaux cultives in vitro. Ann. Sci. Nat. Bot. Biol. Veg. 14:1–223; 1953.
Johansson, M.; Kronestedt-Robards, E. C.; Robards, A. W. Rose leaf structure in relation to different stages of micropropagation. Protoplasma 166:165–176; 1992.
Kozai, T.; Fujiwara, M.; Nayashi, J.; Aitken-Christie, J. The in vitro environment and its control in micropropagation. In: Kurata, K.; Kozai, T., eds Transplant production systems. Dordrecht: Kluwer Academic Publishers; 1992:247–282.
Kunst, L.; Samucls, A. L. Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 43:51–80; 2003.
Louro, R. P.; Dos Santos, A. V.; Machado, R. D.: Ultrastructure of Eucalyptus grandis × Eucalyptus arophylla. I. Shoots cultivated in vitro in multiplication and elongation-rooting media. Int J. Plant Sci. 160:217–227; 1999.
Majada, J. P.; Centeno, M. L.; Feito, L.; Fernández, B.; Sánchez-Tamez, R. Stomatal and cuticular traits on carnation tissue culture under different ventilation conditions. Plant Growth Reg. 25:113–121; 1998.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Noc, N.; Bonini, L. Leaf anatomy of highbush blucberry grown in vitro and during acclimatization to ex vitro conditions. Biol. Plant. 38:19–25; 1996.
Pospišilová J.; Tichá, L.; Kadleček, P.; Haisel, D.; Plazáková, Š. Acclimatization of micropropagated plants to ex vitro conditions. Biol. Plant. 42:481–497; 1999.
Radin, J. W.; Hendrix, D. L. The apoplastic pool of abscisic acid in cotton leaves in relation to stomatal closure. Planta 174:180–186; 1988.
Rossi, V.; De Paoli, G. Micropropagation of artichoke (Cynara scolymus L.). In: Bajaj Y. P. S., ed. Biotechnology in agriculture and forestry: high-tech and micropropagation II, vol. 19, Berlin: Springer-Verlag; 1992:118–134.
Salminen, L.; Uosukainen, M.; Mattsson, P.; Korpela, T. Action of cyclodextrins on germinating seeds and on micropropagated plants. Starch 42:350–353; 1990.
Sha Valli Khan P. S.; Evers, D.; Hausman, J. F. Stomatal characteristics and water relations of in vitro grown Quercus robur NL 100 in relation to acclimatization. Silvac Genet. 48:83; 1999.
Tichá, I.; Radochová, B.; Kadleček, P. Stomatal morphology during acclimatization of tobacco plantlets to ex vitro conditions. Biol. Plant. 42:469–474; 1999.
Voráćková, Z.; Lipavská, H.; Konenéý, P. The efficiency of transfer of plants cultivated in vitro to ex vitro conditions as affected by sugar supply. Biol. Plant. 41:507–515; 1998.
Willmer, C. M. Los estomas [The stomata]. Buenos Aires: Edit. Librería Agropecuaria; 1986.
Zobayed, S. M. A.; Armstrong, J.; Armstrong, W. Leaf anatomy of in vitro tobacco and cauliflower plantlets as affected by different types of ventilation. Plant Sci. 161:537–538; 2001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Apóstolo, N.M., Brutti, C.B. & Llorente, B.E. Leaf anatomy of Cynara scolymus L. in successive micropropagation stages. In Vitro Cell.Dev.Biol.-Plant 41, 307–313 (2005). https://doi.org/10.1079/IVP2004606
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2004606