Abstract
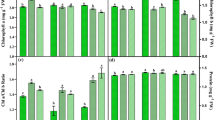
Crepis japonica (L.) D.C. (Asteraceae), a weed with antioxidant, antiallergenic, antiviral and antitumor properties displays both medicinal properties and nutritional value. This study aims to assess the effects of a supplementation of blue light and UV-A radiation on the growth, leaf anatomical structure and phenolic profile of the aerial parts of Crepis japonica. Plants were grown under two light treatments: W (control - white light), W + B (white light supplemented with blue light) and W + UV-A (white light supplemented with UV-A radiation). We recorded the length, width, and weight of fresh and dry leaves, the thickness of the epidermis and mesophyll, and stomata density. The phenolic profiles of the aqueous extracts of the aerial parts were analyzed by HPLC-DAD. There was an increase in the leaf size, stomatal density, and phenolic production, and a thickening of the mesophyll and epidermis. UV-A radiation increased the phenolic production more than blue light. Blue light and UV-A radiation both improved the production of caffeic acid by about 6 and 3 times, respectively, in comparison to control. This compound was first reported as a constituent of the extract from the aerial parts together with caftaric acid. UV-A also promoted the production of chlorogenic acid (about 1.5 times in comparison to the control). We observed that the morphological and chemical parameters of C. japonica are modified in response to blue light and UV-A radiation, which can be used as tools in the cultivation of this species in order to improve its medicinal properties and nutritional value.
Similar content being viewed by others
References
M. B. Garden, Tropicos.org., http://www.tropicos.org (accessed October 2015)
K. Nakamura Y. Kono C. Huang Jr. K. F. Chung and C. I. Peng, Correction of confusions regarding the identity and synonymy of Youngia (Asteraceae: Tribe Cichorieae) in Taiwan, Syst. Bot., 2013, 38 2, 507–516.
J. Y. Lee M. Cha M. R. Kim K. Lee S. Choi and S. Y. Ryu, Two Novel Guaiane Sesquiterpenes from the Whole Plant of Youngia japonica, Planta Med. Lett., 2015, 2, e31–e34. http://www.thieme-connect.de/products
H. Lorenzi, Plantas daninhas do Brasil: terrestres, parasitas, aquáticas e tóxicas, Plantarum, Nova Odessa, 2008
A. C. Slanis and M. C. Perea, Youngia japonica (Asteraceae, Lactuceae), una novedad para la Flora adventicia de Argentina, Bol Soc Argent Bot, 2011, 46 1-2, 139–143.
E. Yae S. Yahara M. El-Aasr T. Ikeda H. Yoshimitsu C. Masuoka M. Ono I. Hide Y. Nakata and T. Nohara, Studies on the constituents of whole plants of Youngia japonica, Chem. Pharm. Bull., 2009, 57 7, 719–723.
L. S. M. Ooi H. Wang Z. He and V. E. C. Ooi, Antiviral activities of purified compounds from Youngia japonica (L.) DC (Asteraceae, Compositae), J. Ethnopharmacol., 2006, 106 2, 187–191.
L. S. M. Ooi H. Wang C. W. LUK and V. E. C. Ooi, Anticancer and antiviral activities of Youngia japonica, (L.) DC (Asteraceae, Compositae), J. Ethnopharmacol., 2004, 94 1, 117–122.
W. Chen Q. Liu J. Wang J. Zou D. Meng J. Zuo X. Zhu and W. Zhao, New guaiane, megastigmane and eudesmane-type sesquiterpenoids and anti-inflammatory constituents from Youngia japonica, Planta Med., 2006, 72 2, 143–150.
S. Taba J. Sawada and Z. Moromizato, Nematicidal activity of Okinawa Island plants on the root-knot nematode Meloidogyne incognita (Kofoid and White) Chitwood, Plant Soil, 2008, 303 1-2, 207–216.
X. C. Liu Q. Liu X. B. Chen Q. Z. Liu and Z. L. Liu, Larvicidal activity of the essential oil of Youngia japonica aerial parts and its constituents against Aedes albopictus, Z. Naturforsch., C: Biochem., Biophys., Biol., Virol., 2015, 70 1-2, c1–c6 10.1515/znc-2014-4074.
R. J. Robbins, Phenolic Acids in Foods: An Overview of Analytical Methodology, J. Agric. Food Chem., 2003, 51, 2866–2887.
K. Krak and P. Mráz, Trichomes in the tribe Lactuceae (Asteraceae)—taxonomic implications, Biologia, 2008, 63 5, 616–630.
L. S. Santos H. S. Dariva R. H. Muller O. J. G. Almeida and L. A. Souza, Seedling structure in Asteraceae weedy species: considerationson the vascular system, Braz. J. Bot., 2014, 37 4, 631–635.
H. Smith, Light quality, photo perception, and plant strategy, Ann. Rev. Plant Physiol., 1982, 33, 481–518.
L. Huché-Thélier L. Crespel J. Le Gourriere P. Morela S. Sakr and N. Leduc, Light signaling and plant responses to blue and UV radiations— Perspectives for applications in horticulture, Environ. Exp. Bot., 2016, 121, 22–38.
Q. Li and C. Kubota, Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce, Environ. Exp. Bot., 2009, 67 1, 59–64.
L. B. S. Nascimento M. V. Leal-Costa M. A. S. Coutinho N. S. Moreira C. L. S. Lage N. S. Barbi S. S. Costa and E. S. Tavares, Increased antioxidant activity and changes in phenolic profile of Kalanchoe pinnata (Lamarck) Persoon (Crassulaceae) specimens grown under supplemental blue light, Photochem. Photobiol., 2013, 89, 391–399.
A. C. Schuerger C. S. Brown and E. C. Stryjewski, Anatomical features of pepper plants (Capsicum annuum L.) grown under red light-emitting diodes supplemented with blue or far-red light, Ann. Bot., 1997, 79 3, 273–282.
F. Rapparini A. Rotondi and R. Baraldi, Blue light regulation of the growth of Prunus persica plants in a long term experiment: morphological and histological observations, Trees, 1999, 14 3, 169–176.
M. V. Leal-Costa L. B. S. Nascimento N. S. Moreira F. Reinert S. S. Costa C. L. Lage and E. S. Tavares, Influence of blue light on the leaf morphoanatomy of in vitro Kalanchoe pinnata (Lamarck) Persoon (Crassulaceae), Microsc. Microanal., 2010, 16 5, 576–582.
A. F. Macedo M. V. Leal-Costa E. S. Tavares C. L. S. Lage and M. A. Esquibel, The effect of light quality on leaf production and development of in vitro-cultured plants of Alternanthera brasiliana Kuntze, Environ. Exp. Bot., 2011, 70 1, 43–50.
C. P. Victório M. V. Leal-Costa E. S. Tavares R. M. Kuster and C. L. S. Lage, Effects of Supplemental UV-A on the Development, Anatomy and Metabolite Production of Phyllanthus tenellus Cultured In Vitro, Photochem. Photobiol., 2011, 87 3, 685–689.
T. O’Brien N. Feder and M. E. McCully, Polychromatic staining of plant cell walls by toluidine blue O, Protoplasma, 1964, 59 2, 368–373.
D. A. Johansen, Plant microtechnique, McGraw-Hill Book Company, New York-London, 1940
L. M. Casanova D. da Silva M. Sola - Penna L. M. Camargo D. M. Celestrini L. W. Tinoco and S. S. Costa, Identification of chicoric acid as a hypoglycemic agents from Ocimum gratissimum leaf extract in a biomonitoring in vivo study, Fitoterapia, 2014, 93, 132–141.
B. Glowacka, The effect of blue light on the height and habit of the tomato (Lycopersicon esculentum Mill.) transplant, Folia Hortic., 2004, 16 2, 3–10.
A. Wozny and M. Jerzy, Effect of light wavelength on growth and flowering of narcissi forced under short-day and low quantum irradiance conditions, J. Hortic. Sci. Biotechnol., 2007, 82 6, 924–928.
S. Singh, A. Dube and P. Gupta, Fluorescence study of maize irradiated by UVA, Pure Appl. Opt., 7(3), L39.
T. Tezuka T. Hotta and I. Watanabe, Growth promotion of tomato and radish plants by solar UV radiation reaching the Earth’s surface, J. Photochem. Photobiol., B, 1993, 19 1, 61–66.
F. Antonelli D. Grifoni F. Sabatini and G. Zipoli, Morphological and physiological responses of bean plants to supplemental UV radiation in a Mediterranean climate, Plant Ecol., 1997, 128 1-2, 127–136.
S. H. Sarghein J. Carapetian and J. Khara, The effects of UV radiation on some structural and ultrastructural parameters in pepper (Capsicum longum, A. DC.), Turk. J. Biol., 2011, 35, 69–77.
A. Manukyan, Effects of PAR and UV-B radiation on herbal yield, bioactive compounds and their antioxidant capacity of some medicinal plants under controlled environmental conditions, Photochem. Photobiol., 2013, 89 2, 406–414.
M. Veit D. Strack F. Czygan V. Wray and L. Witte, Di-e-caffeoyl-meso-tartaric acid in the barren sprouts of Equisetum arvense, Phytochemistry, 1991, 30 2, 527–529.
A. P. Sobolev, Elvino Brosio, Raffaella Gianferri and Anna L. Segre, Metabolic profile of lettuce leaves by high-field NMR spectra, Magn. Reson. Chem., 2005, 43, 625–638.
M. Bahri P. Hance S. Grec M. Quillet F. Trotin J. Hilbert and T. Hendriks, A “Novel” Protocol for the Analysis of Hydroxycinnamic Acids in Leaf Tissue of Chicory (Cichorium intybus L., Asteraceae), Sci. World J., 2012, 2012, 142983.
M. A. Farag S. M. Ezzat M. M. Salama and M. G. Tadros, Anti-acetylcholinesterase potential and metabolome classification of 4 Ocimum species as determined via UPLC/qTOF/MS and chemometrictools, J. Pharm. Biomed. Anal., 2016, 125, 292–302.
B. S. Khoza S. Gbashi P. A. Steenkamp P. B. Njobeh and N. E. Madala, Identification of hydroxylcinnamoyl tartaric acid esters in Bidens pilosa by UPLC-tandem mass spectrometry, S. Afr. J. Bot., 2016, 103, 95–100.
A. S. Meyer J. L. Donovan D. A. Pearson A. L. Waterhouse and E. N. Frankel, Fruit Hydroxycinnamic Acids Inhibit Human Low-Density Lipoprotein Oxidation in Vitro, J. Agric. Food Chem., 1998, 46, 1783–1787.
M. C. Búfalo I. Ferreira G. Costa V. Francisco J. Liberal M. T. Cruz M. C. Lopes M. T. Batista and J. M. Sforcin, Propolis and itsconstituent caffeic acid suppress LPS-stimulated pro-inflammatory response by blocking NF-?B andMAPK activation inmacrophages, J. Ethnopharmacol., 2013, 149, 84–92.
E. Fuentes and I. Palomo, Mechanisms of endothelial cell protection by hydroxycinnamic acids, Vasc. Pharmacol., 2014, 63, 155–161.
M. A. Alam N. Subhan H. Hossain M. Hossain H. M. Reza M. M. Rahman and M. O. Ullah, Hydroxycinnamic acid derivatives: a potential class of natural compounds for the management of lipid metabolism and obesity, Nutr. Metab., 2016, 13–27.
N. Calabriso E. Scoditti M. Massaro M. Pellegrino C. Storelli I. Ingrosso G. Giovinazzo and M. A. Carluccio, Multiple antiinflammatory and antiatherosclerotic properties of red wine polyphenolic extracts: differential role of hydroxycinnamic acids, flavonols and stilbenes on endothelial inflammatory gene expression, Eur. J. Nutr., 2016, 55, 477–489.
N. Liang and D. D. Kitts, Role of Chlorogenic Acids in Controlling Oxidative and inflammatory Stress Conditions, Nutrients, 2016, 8 1, 16.
R. Vinayagam M. Jayachandran and B. Xu, Antidiabetic Effects of Simple Phenolic Acids: A Comprehensive Review, Phytother. Res., 2016, 30, 184–199.
Z. Zhang X. Li Y. Chu M. Zhang Y. Wen C. Duan and Q. Pan, Three types of ultraviolet irradiation differentially promote expression of shikimate pathway genes and production of anthocyanins in grape berries, Plant Physiol. Biochem., 2012, 57, 74–83.
K. Taulavuori V. Hyöky J. Oksanen E. Taulavuori and R. Julkunen-Tiitto, Species-specific differences in synthesis of flavonoids and phenolic acids under increasing periods of enhanced blue light, Environ. Exp. Bot., 2016, 121, 145–150.
U. J. Jung M. Lee Y. B. Park S. Jeon and M. Choi, Antihyperglycemic and Antioxidant Properties of Caffeic Acid in db/db Mice, J. Pharmacol. Exp. Ther., 2006, 318 2, 476–483.
K. Ikeda K. Tsujimoto M. Uozaki M. Nishide Y. Suzuki A. H. Koyama and H. Yamasaki, Inhibition of multiplication of herpes simplex virus by caffeic acid, International, J. Mol. Med., 2011, 28, 595–598.
N. R. Prasad A. Karthikeyan S. Karthikeyan and B. V. Reddy, Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line, Mol. Cell. Biochem., 2011, 349, 11–19.
E. Tsormpatsidis R. G. C. Henbest F. J. Davis N. H. Battey P. Hadley and A. Wagstaffe, UV irradiance as a major influence on growth, development and secondary products of commercial importance in Lollo Rosso lettuce ‘Revolution’ grown under polyethylene films, Environ. Exp. Bot., 2008, 63, 232–239.
G. Agati C. Brunetti M. Di Ferdinando F. Ferrini S. Pollastri and M. Tattini, Functional roles of flavonoids in photoprotection: New evidence, lessons from the past, Plant Physiol. Biochem., 2013, 72, 35–45.
T. Kotilainen R. Tegelberg R. Julkunen-Tiittoz A. Lindfors and P. J. Aphalo, Metabolite specific effects of solar UV-A and UV-B on alder and birch leaf phenolics, Glob. Chang. Biol., 2008, 14, 1–11.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Constantino, L.F.d.S., Nascimento, L.B.d.S., Casanova, L.M. et al. Responses of Crepis japonica induced by supplemental blue light and UV-A radiation. Photochem Photobiol Sci 16, 238–245 (2017). https://doi.org/10.1039/c6pp00343e
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c6pp00343e