Abstract
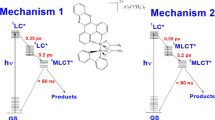
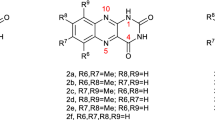
The electronic properties of vemurafenib (VB) provide a rational basis for understanding its strong UVA-induced phototoxicity. Thus, solvation of hydrophobic VB by hydrogen bonding solvents controls its photophysical, photochemical and photosensitizing properties. Addition of phosphate buffered saline (PBS) to methanol (MeOH) induces a bathochromic shift of the VB absorbance spectrum and a fluorescence emission (λmax = 450 nm, quantum yield (Φ) = 0.011). Phosphorescence (λmax = 461 nm) is observed at 77 K in MeOH. 308 nm laser flash spectroscopy demonstrates that the lifetimes (τ) and quantum yields of the VB triplet state in deaerated MeOH (τMeOH = 0.41 μs, λmax ∼ 380 nm), MeOH–PBS and HSA solutions markedly depend on the microenvironment. A long-lived radical (half-life >200 μs) is also formed. The state is quenched by O2 and electron donors (Cys and 2′-deoxyguanosine) at a rate constant >1 × 109 M−1 s−1. UVA-irradiation of VB in air-saturated MeOH or MeOH–PBS solutions produces a UVA-absorbing photoproduct (Φ ∼ 5 × 10−4). VB photosensitizes Trp destruction by type I (radical formation) and type II (singlet oxygen (1O2) formation) photodynamic reactions (Φ = 0.005). Singlet oxygen production is further demonstrated by the VB-photosensitized His oxidation (ΦMeOH = 0.006).
Similar content being viewed by others
References
J. T. Lee, L. Li, P. A. Brafford, M. van den Eijnden, M. B. Halloran, K. Sproesser, N. K. Haass, K. S. Smalley, J. Tsai, G. Bollag, M. Herlyn, Pigm. Cell Melanoma Res., 2010, 23, 820–827.
P. B. Chapman, A. Hauschild, C. Robert, J. B. Haanen, P. Ascierto, J. Larkin, R. Dummer, C. Garbe, A. Testori, M. Maio, D. Hogg, P. Lorigan, C. Lebbe, T. Jouary, D. Schadendorf, A. Ribas, S. J. O’Day, J. A. Sosman, J. M. Kirkwood, A. M. Eggermont, B. Dreno, K. Nolop, J. Li, B. Nelson, J. Hou, R. J. Lee, K. T. Flaherty, G. A. McArthur, N. Engl. J. Med., 2011, 364, 2507–2516.
G. A. McArthur, P. B. Chapman, C. Robert, J. Larkin, J. B. Haanen, R. Dummer, A. Ribas, D. Hogg, O. Hamid, P. A. Ascierto, C. Garbe, A. Testori, M. Maio, P. Lorigan, C. Lebbe, T. Jouary, D. Schadendorf, S. J. O’Day, J. M. Kirkwood, A. M. Eggermont, B. Dreno, J. A. Sosman, K. T. Flaherty, M. Yin, I. Caro, S. Cheng, K. Trunzer, A. Hauschild, Lancet Oncol., 2014, 15, 323–332.
R. Dummer, J. Rinderknecht, S. M. Goldinger, N. Engl. J. Med., 2012, 366, 480–481.
P. Gelot, H. Dutartre, A. Khammari, A. Boisrobert, C. Schmitt, J. C. Deybach, J. M. Nguyen, S. Seite, B. Dreno, Exp. Dermatol., 2013, 22, 297–298.
OECD, OECD Guidelines for the Testing of Chemicals, Section, 2004, vol, 4.
FDA/Center for Drug Evaluation and Research, Division of Drug Oncology products (HFD-150), 2011.
C. Brugière, A. Stefan, C. Morice, E. Cornet, A. Moreau, S. Allouche, L. Verneuil, Br. J. Dermatol., 2014, 171, 1529–1532.
E. Kohen, R. Santus and J. G. Hirschberg, Photobiology, Academic Press, San Diego, 1995.
A. Sharma, S. R. Shah, H. Illum, J. Dowell, Drugs, 2012, 72, 2207–2222.
C. A. Parker, Photoluminescence of Solutions, Elsevier, Amsterdam, 1968.
I. Tirache, P. Morlière, Redox Rep., 1995, 1, 105–111.
W. A. Waterval, J. L. Scheijen, M. M. Ortmans-Ploemen, C. D. Habets-van der Poel, J. Bierau, Clin. Chim. Acta, 2009, 407, 36–42.
J. K. Kamal, L. Zhao, A. H. Zewail, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 13411–13416.
S. M. Boudon, U. Plappert-Helbig, A. Odermatt, D. Bauer, Toxicol. Sci., 2014, 137, 259–267.
E. G. Hohenstein, C. D. Sherrill, J. Phys. Chem. A, 2009, 113, 878–886.
Protein Data Bank.
V. Lhiaubet-Vallet, F. Bosca, M. A. Miranda, J. Phys. Chem. B, 2007, 111, 423–431.
R. Battino, T. R. Rettich, T. Tominaga, J. Phys. Chem. Ref. Data, 1983, 12, 163–168.
A. Guha, D. Panda, Int. J. Chem. Sci., 2008, 6, 1147–1167.
S. Steenken, S. V. Jovanovic, J. Am. Chem. Soc., 1997, 119, 617–618.
P. Morlière, F. Bosca, M. A. Miranda, J. V. Castell, R. Santus, Photochem. Photobiol., 2004, 80, 535–541.
A. A. Gorman, L. Hamblett, M. A. J. Rodgers, J. Am. Chem. Soc., 1984, 106, 4679–4682.
C. Chahidi, M. Aubailly, A. Momzikoff, M. Bazin, R. Santus, Photobiochem. Photobiophys., 1981, 33, 641–649.
G. Winter, H. Shioyama, U. Steiner, Chem. Phys. Lett., 1981, 81, 547–552.
M. Bazin, L. K. Patterson, R. Santus, J. Phys. Chem., 1983, 87, 189–190.
K. Gollnick, in Radiation Research: Biochemical, Chemical and Physical Perspectives, ed. O. F. Nygaard, H. I. Adler and W. D. Sinclair, Academic Press, New York, 1975, pp. 590–611.
F. Wilkinson, J. G. Brummer, J. Phys. Chem. Ref. Data, 1981, 10, 809–999.
A. Salvador, D. Vedaldi, P. Brun, S. Dall’Acqua, Toxicol. in Vitro, 2014, 28, 803–811.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morlière, P., Boscá, F., Silva, A.M.S. et al. A molecular insight into the phototoxic reactions observed with vemurafenib, a first-line drug against metastatic melanoma. Photochem Photobiol Sci 14, 2119–2127 (2015). https://doi.org/10.1039/c5pp00231a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c5pp00231a