Abstract
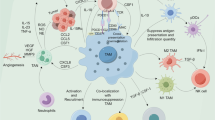
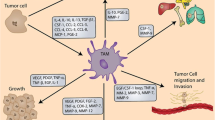
Macrophages are one of the principal host cell populations in solid tumors. They are capable, due to their plasticity, of acquiring phenotypes that either combat (M1 type) or promote (M2 type) neoplastic growth. These cells, known as tumor-associated macrophages (TAMs), play complex but pivotal roles in the outcome of photodynamic therapy (PDT) of malignant lesions. Among the various parenchymal and stromal cell populations found in tumors, TAMs have been shown to have the greatest capacity for the uptake of systemically administered photosensitizers. Both the tumor-localizing property of photosensitizers and their tumor-localized fluorescence could be partly attributed to the activity of TAMs. Since resident TAMs with accumulated high photosensitizer content will sustain high degrees of PDT damage, this population (predominantly M2 in most tumors) is selectively destroyed, and during the ensuing inflammatory reaction is replaced with newly invading macrophages of M1 phenotype. These macrophages are sentinels responding to DAMP signals from PDT-treated tumor cells and in turn are mobilized to generate a variety of inflammatory/immune mediators and opsonins. They have a critical role in contributing to the therapeutic effect of PDT by mediating disposal of killed cancer cells and by processing/presenting tumor antigens to T lymphocytes. However, TAMs accumulating in the later post-PDT phase can acquire the M2 (healing) phenotype, and could have a role in tumor recurrence by releasing factors that promote angiogenesis and the survival/proliferation of remaining cancer cells. Various therapeutic strategies modulating TAM activity in the PDT response have potential for clinical use for improving PDT-mediated tumor control.
Similar content being viewed by others
References
H. Hewald, A. Egesten, The Janus face of macrophages in immunity, J. Innate Immun., 2014, 6, 713–715.
C. D. Mills, K. Ley, M1 and M2 macrophages: the chicken and the egg of immunity, J. Innate Immun., 2014, 6, 716–726.
P. J. Murray, T. A. Wynn, Protective and pathogenic functions of macrophage subsets, Nat. Rev. Immunol., 2011, 11, 723–737.B.
F. Geissmann, M. G. Manz, S. Jung, M. H. Sieweke, M. Merad, K. Ley, Development of monocytes, macrophages, and dendritic cells, Science, 2010, 327, 656–661.
M. R. Gwinn, V. Vallyathan, Respiratory burst: role in signal transduction in alveolar macrophages, J. Toxicol. Environ. Health, Part B, 2006, 9, 27–39.
N.-B. Hao, M.-H. Lü, Y.-H. Fan, Y.-L. Cao, Z.-R. Zhang, S.-M. Yang, Macrophages in tumor microenvironments and the progression of tumors, Clin. Dev. Immunol., 2012, 2012, 948098.
M. Korbelik, PDT-associated host response and its role in the therapy outcome, Lasers Surg. Med., 2006, 38, 500–508.
A. P. Castano, P. Mroz, M. R. Hamblin, Photodynamic therapy and anti-tumor immunity, Nat. Rev. Cancer, 2006, 6, 535–545.
M. Korbelik and G. Krosl, Photosensitizer distribution and photosensitized damage of tumor tissue, in The fundamental bases of phototherapy, ed. H. Hönigsmann, G. Jori and A. R. Young, OEMF spa, Milan, 1996, pp. 229–245.
M. R. Hamblin, E. L. Newman, On the mechanism of tumor-localizing effect in photodynamic therapy, J. Photochem. Photobiol., B., 1994, 23, 3–8.
P. J. Bugelski, C. W. Porter, T. J. Dougherty, Autoradiographic distribution of hematoporphyrin derivative in normal and tumor tissue of the mouse, Cancer Res., 1981, 41, 4606–4612.
W. S. Chan, J. F. Marshall, G. Y. F. Lam, I. R. Hart, Tissue uptake, distribution, and potency of the photoactivable dye chloraluminum sulfonated phthalocyanine in mice bearing transplantable tumors, Cancer Res., 1988, 48, 3040–3044.
B. W. Henderson, D. A. Bellnier, Tissue localization of photosensitizers and the mechanism of photodynamic tissue destruction, CIBA Found. Symp., 1989, 146, 112–125.
M. Korbelik, G. Krosl, D. J. Chaplin, Photofrin uptake by murine macrophages, Cancer Res., 1991, 51, 2251–2255.
M. Korbelik, G. Krosl, H. Adomat, K. A. Skov, The effect of differentiation on photosensitizer uptake by HL60 cells, Photochem. Photobiol., 1993, 58, 670–675.
M. Korbelik, G. Krosl, P. L. Olive, D. J. Chaplin, Distribution of Photofrin between tumour cells and tumour associated macrophages, Br. J. Cancer, 1991, 64, 508–512.
M. Korbelik, Distribution of disulfonated and tetrasulfonated aluminum phthalocyanine between malignant and host cell populations of murine fibrosarcoma, J. Photochem. Photobiol., B, 1993, 20, 173–181.
M. Korbelik, G. Krosl, Accumulation of benzoporphyrin derivative in malignant and host cell populations of the murine RIF tumor, Cancer Lett., 1995, 97, 249–254.
M. Korbelik, G. Krosl, Photofrin accumulation in malignant and host cell populations of various tumors, Br. J. Cancer, 1996, 73, 506–513.
M. Korbelik, G. Krosl, Cellular levels of photosensitizers in tumours: the role of proximity to the blood supply, Br. J. Cancer, 1994, 70, 604–610.
G. Jori, In vivo transport and pharmacokinetic behaviour of tumor photosensitizers, CIBA Found. Symp., 1989, 146, 78–86.
M. Korbelik, G. Krosl, D. J. Chaplin, Can PDT be potentiated by immunotherapy?, Proc. SPIE-Int. Soc. Opt. Eng., 1991, 1616, 192–198.
M. R. Hamblin, J. L. Miller, B. Ortel, Scavenger-receptor targeted photodynamic therapy, Photochem. Photobiol., 2000, 72, 533–540.
M. R. Hamblin, J. L. Miller, I. Rizvi, B. Ortel, Degree of substitution of chlorin e6 on charged poly-L-lysine chains affects their cellular uptake, localization and phototoxicity towards macrophages and cancer cells, J. X-ray Sci. Technol., 2002, 10, 139–152.
J. F. Marshall, W.-S. Chan, I. R. Hart, Effect of photodynamic therapy on anti-tumor immune defenses: comparison of the photosensitizers hematoporphyrin derivative and chloro-aluminum sulfonated phthalocyanine, Photochem. Photobiol., 1989, 49, 627–632.
R. W. Steubing, S. Yeturu, A. Tuccillo, C.-H. Sun, M. W. Berns, Activation of macrophages by Photofrin II during photodynamic therapy, J. Photochem. Photobiol., B, 1991, 10, 133–145.
N. Yamamoto, J. K. Hoober, N. Yamamoto, S. Yamamoto, Tumoricidal capacities of macrophages photodynamically activated with hematoporphyrin derivative, Photochem. Photobiol., 1992, 56, 245–250.
S. Evans, W. Matthews, R. Perry, D. Fraker, J. Norton, H. I. Pass, Effect of photodynamic therapy on tumor necrosis factor production by murine macrophages, J. Natl. Cancer Inst., 1990, 82, 34–39.
M. Korbelik, J. Sun, I. Cecic, Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response, Cancer Res., 2005, 65, 1018–1026.
F. Zhou, D. Xing, W. R. Chen, Regulation of HSP70 on activating macrophages using PDT-induced apoptotic cells, Int. J. Cancer, 2009, 125, 1380–1389.
S. Song, F. Zhou, W. R. Chen, D. Xing, PDT-induced HSP70 externalization up-regulates NO production via TLR2 signal pathway in macrophages, FEBS Lett., 2013, 587, 128–135.
B. Stott, M. Korbelik, Activation of complement C3, C5, and C9 genes in tumors treated by photodynamic therapy, Cancer Immunol. Immunother., 2007, 56, 649–658.
S. Merchant, J. Sun, M. Korbelik, Dying cells program their expedient disposal: serum amyloid P component upregulation in vivo and in vitro induced by photodynamic therapy of cancer, Photochem. Photobiol. Sci., 2007, 6, 1284–1289.
M. Korbelik, J. Banáth, W. Zhang, F. Wong, J. Bielawski, D. Separovic, Ceramide and sphingosine-1-phosphate/sphingosine act as photodynamic therapy-elicited damage-associated molecular patterns: Release from cells and impact on tumor-associated macrophages, J. Anal. Bioanal. Tech., 2014, S1, 009. 10.4172/2155-9872.S1-009
M. Korbelik, Complement upregulation in photodynamic therapy treated tumors: role of Toll-like receptor pathway and NFκB, Cancer Lett., 2009, 281, 232–238.
I. Cecic, J. Sun, M. Korbelik, Role of complement anaphylatoxin C3a in photodynamic therapy-elicited engagement of neutrophils and other immune cells, Photochem. Photobiol., 2006, 82, 558–562.
A. J. Nauta, M. R. Daha, C. van Kooten, A. Roos, Recognition and clearance of apoptotic cells: a role of complement and pentraxins, Trends Immunol., 2003, 24, 148–154.
S. L. Cassel, F. S. Sutterwala, Sterile inflammatory responses mediated by the NLRP3 inflammasome, Eur. J. Immunol., 2010, 40, 607–611.
G. Krosl, M. Korbelik, G. J. Dougherty, Induction of immune cell infiltration into murine SCCVII tumour by Photofrin-based photodynamic therapy, Br. J. Cancer, 1995, 71, 549–555.
M. Korbelik, Tumor-localized insult delivered by photodynamic therapy and the breakdown of tumor immunotolerance, in Tumor Ablation, ed. Y. Keisari, Springer, Dordrecht, 2013, vol. 5, (The Tumor Microenvironment series), ch. 7, pp.121–132.
M. Korbelik, G. Krosl, Enhanced macrophage cytotoxicity against tumor cells treated with photodynamic therapy, Photochem. Photobiol., 1994, 60, 497–502.
S. K. Biswas, P. Allavena, A. Mantovani, Tumor-associated macrophages: functional diversity, clinical significance, and open questions, Semin. Immunopathol., 2013, 35, 585–600.
Q. Liu, M. R. Hamblin, Macrophage-targeted photodynamic therapy: scavenger receptor expression and activation state, Int. J. Immunopathol. Pharmacol., 2005, 18, 391–402.
J. Canton, D. Neculai, S. Grinstein, Scavenger receptors in homeostasis and immunity, Nat. Rev. Immunol., 2013, 13, 621–634.
T. N. Demidova, M. R. Hamblin, Macrophage-targeted photodynamic therapy, Int. J. Immunopathol. Pharmacol., 2004, 17, 117–126.
F. Anatelli, P. Mroz, Q. Liu, A. P. Castano, E. Swietlik, M. R. Hamblin, Macrophage-targeted photosensitizer conjugate delivered by intratumoral injection, Mol. Pharm., 2006, 3, 654–664.
A. Tawakol, A. P. Castano, F. Anatelli, G. Bashian, J. Stern, T. Zahra, F. Gad, S. Chirico, A. Ahmadi, A. J. Fischman, J. E. Muller, M. R. Hamblin, Photosensitizer delivery to vulnerable atherosclerotic plaque: comparison of macrophage-targeted conjugate versus free chlorin(e6), J. Biomed. Opt., 2006, 11, 021008.
A. Tawakol, A. P. Castano, F. Gad, T. Zahra, G. Bashian, R. Q. Migrino, A. Ahmadi, J. Stern, F. Anatelli, S. Chirico, A. Shirazi, S. Syed, A. J. Fischman, J. E. Muller, M. R. Hamblin, Intravascular detection of inflamed atherosclerotic plaques using a fluorescent photosensitizer targeted to the scavenger receptor, Photochem. Photobiol. Sci., 2008, 7, 33–39.
A. J. Gomes, L. O. Lunardi, J. M. Marchetti, C. N. Lunardi, A. C. Tedesco, Photobiological and ultrastructural studies of nanoparticles of poly(lactic-co-glycolic acid)-containing bacteriochlorophyll-a as a photosensitizer useful for PDT treatment, Drug Delivery, 2005, 12, 159–164.
H. Kim, Y. Kim, I.-H. Kim, K. Kim, Y. Choi, ROS-responsive activable photosensitizing agent for imaging and photodynamic therapy of activated macrophages, Theranostics, 2014, 4, 1–11.
J. Klesing, A. Wiehe, B. Gitter, S. Gräfe, M. Epple, Positively charged calcium phosphate/polymer nanoparticles for photodynamic therapy, J. Mater. Sci. Mater. Med., 2010, 21, 997–892.
M. Korbelik, V. R. Naraparaju, N. Yamamoto, Macrophage directed immunotherapy as adjuvant to photodynamic therapy of cancer, Br. J. Cancer, 1997, 75, 202–207.
G. Krosl, M. Korbelik, Potentiation of photodynamic therapy by immunotherapy: The effect of Schizophyllan (SPG), Cancer Lett., 1994, 84, 43–49.
M. Uehara, K. Sano, Z.-L. Wang, J. Sekine, H. Ikeda, T. Inokuchi, Enhancement of the photodynamic antitumor effect by streptococcal preparation OK-432 in the mouse carcinoma, Cancer Immunol. Immunother., 2000, 49, 401–409.
R. C. Myers, B. H. Lau, D. Y. Kunihira, R. R. Torrey, J. L. Woolley, J. Tosk, Modulation of hematoporphyrin derivative-sensitized phototherapy with corynebacterium parvum in murine transitional cell carcinoma, Urology, 1989, 33, 230–235.
M. Korbelik, J. Sun, J. J. Posakony, Interaction Between Photodynamic Therapy and BCG Immunotherapy Responsible for the Reduced Recurrence of Treated Mouse Tumors, Photochem. Photobiol., 2001, 73, 403–409.
M. Korbelik, I. Cecic, Enhancement of tumour response to photodynamic therapy by adjuvant mycobacterium cell-wall treatment, J. Photochem. Photobiol., B, 1998, 44, 151–158.
M. Korbelik, S. Merchant, N. Huang, Exploitation of immune response-eliciting properties of hypocrellin photosensitizer SL052-based photodynamic therapy for eradication of malignant tumors, Photochem. Photobiol., 2009, 85, 1418–1424.
G. Krosl, M. Korbelik, J. Krosl, G. J. Dougherty, Potentiation of Photodynamic therapy elicited antitumor response by localized treatment with granulocyte-macrophage colony stimulating factor, Cancer Res., 1996, 56, 3281–3286.
M. Korbelik, G. J. Dougherty, Complement activation approaches for use in conjunction with PDT for cancer treatment, Proc. SPIE-Int. Soc. Opt. Eng., 2005, 5695, 17–26.
M. Korbelik, J. Sun, I. Cecic, K. Serrano, Adjuvant treatment for complement activation increases the effectiveness of photodynamic therapy of solid tumors, Photochem. Photobiol. Sci., 3004, 3, 812–816.
M. Korbelik, P. D. Cooper, Potentiation of photodynamic therapy of cancer by complement: the effect of γ-inulin, Br. J. Cancer, 2007, 96, 67–72.
M. Korbelik, I. Cecic, Complement activation cascade and its regulation: relevance for the response of solid tumors to photodynamic therapy, J. Photochem. Photobiol., B, 2008, 93, 53–59.
C. J. Gomer, A. Ferrario, M. Luna, N. Rucker, S. Wong, Photodynamic therapy: combined modality approaches targeting the tumor microenvironment, Lasers Surg. Med., 2006, 38, 516–521.
A. Ferrario, C. J. Gomer, Avastin enhances photodynamic therapy treatment of Kaposi’s sarcoma in a mouse tumor model, J. Environ. Path. Toxicol. Oncol., 2006, 25, 251–259.
M. Makowski, T. Grzela, J. Nidrla, M. Azarczyk, P. Mroz, M. Kopee, D. Nowis, P. Mrowka, M. Wasik, M. Jakobisiak, J. Golab, Inhibitor of cyclooxigenase-2 indirectly potentiates antitumor effects of photodynamic therapy in mice, Clin. Cancer Res., 2003, 9, 5417–5422.
A. Ferrario, N. Rucker, S. Wong, M. Luna, C. J. Gomer, Survivin, a member of the Inhibitor of Apoptosis family, is induced by photodynamic therapy and is a target for improving treatment response, Cancer Res., 2007, 67, 4989–4995.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Korbelik, M., Hamblin, M.R. The impact of macrophage-cancer cell interaction on the efficacy of photodynamic therapy. Photochem Photobiol Sci 14, 1403–1409 (2015). https://doi.org/10.1039/c4pp00451e
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c4pp00451e