Abstract
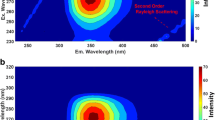
The effect of Hg2+ on the fluorescence intensity of three fulvic acids (Pahokee Peat, Pony Lake and Suwannee River) was studied. The fluorescence intensity decreased in the presence of added Hg2+, while the fluorescence lifetimes were independent of the concentration of Hg2+ in solution. These results are indicative of ground-states association between the fulvic acids and Hg2+ with formation of stable non-fluorescent complexes (static quenching process). The analysis of the excitation–emission matrices with the Singular Value Decomposition (SVD) and Multivariate Curve Resolution–Alternating Least Squares (MCR-ALS) methods provided additional valuable information regarding the binding properties between Hg2+ ions and specific fluorescence components of the fulvic acids. The three fulvic acids were shown to contain the same three groups of fluorophores characterized by excitation/emission pairs in the following regions: (320–330 nm/425–450 nm), (370–375 nm/465–500 nm), (290–295 nm/370–395 nm). These pairs are almost not affected by the change of pH from 2.0 to 7.0. Ryan–Weber and modified Stern–Volmer methods were used to analyze the static fluorescence quenching of the individual components. Similar conditional stability constants of Hg2+ binding for the three components were found by both methods. The obtained log K values are in the range of 4.4 to 5.4.
Similar content being viewed by others
Abbreviations
- DOM:
-
Dissolved organic matter
- EEM:
-
Excitation–emission matrices
- FA:
-
Fulvic acid
- HA:
-
Humic acid
- HS:
-
Humic substances
- IHSS:
-
International Humic Substance Society
- M w:
-
Molecular weight
- MCR-ALS:
-
Multivariate Curve Resolution–Alternating Least Squares
- PP:
-
Pahokke Peat
- PARAFAC:
-
Parallel Factor Analysis
- PL:
-
Pony Lake
- SDV:
-
Singular Value Decomposition
- SR:
-
Suwannee River
References
N. Hertkorn, H. Claus, P. H. Schmitt-Kopplin, E. M. Perdue, Z. Filip, Utilization and transformation of aquatic humic substances by autochthonous microorganisms, Environ. Sci. Technol., 2002, 36, 4334–4345.
S. McDonald, A. G. Bishop, P. D. Prenzler, K. Robards, Analytical chemistry of freshwater humic substances, Anal. Chim. Acta, 2004, 527, 105–124.
P. Janoš, Separation methods in the chemistry of humic substances, J. Chromatogr., A, 2003, 983, 1–18.
T. D. Gauthier, E. C. Shane, W. F. Guerln, W. R. Seilz, C. L. Grant, Fluorescence quenching method for determining equilibrium constants for polycyclic aromatic hydrocarbons binding to dissolved humic materials, Environ. Sci. Technol., 1986, 20, 1162–1166.
F. Fang, S. Kanan, H. H. Patterson, C. S. Cronan, A spectrofluorimetric study of the binding of carbofuran, carbaryl, and aldicarb with dissolved organic matter, Anal. Chim. Acta, 1998, 373, 139–151.
X. Lu, R. Jaffe, Interaction between Hg(ii) and natural dissolved organic matter: a fluorescence spectroscopy based study, Water Res., 2001, 35, 1793–1803.
F. C. Wu, Y. R. Cai, R. D. Evans, P. Dillon, Complexation between Hg(ii) and dissolved organic matter in stream waters: an application of fluorescence spectroscopy, Biogeochemistry, 2004, 71, 339–351.
Y. Cai, R. Jaffe, R. D. Jones, Interactions between dissolved organic carbon and mercury species in surface waters of the Florida Everglades, Appl. Geochem., 1999, 14, 395–407.
J. Wu, H. Zhang, P. J. He, L. M. Shao, Insight into the heavy metal binding potential of dissolved organic matter in MSW leachate using EEM quenching combined with PARAFAC analysis, Water Res., 2011, 45, 1711–1719.
J. C. G. Esteves da Silva, C. J. S. Oliveira, Metal ion complexation properties of fulvic acids extracted from composted sewage sludge as compared to a soil fulvic acid, Water Res., 2002, 36, 3404–3409.
R. J. Yang, C. M. G. van den Berg, Metal complexation by humic substances in sea water, Environ. Sci. Technol., 2009, 43, 7192–7197.
D. Hernandez, C. Plaza, N. Senesi, A. Polo, Detection of copper(ii) and zinc(ii) binding to humic acids from pig slurry and amended soils by fluorescence spectroscopy, Environ. Pollut., 2006, 143, 212–220.
A. M. Martensson, C. Aulin, O. Wahlberg, S. Agren, Effect of humic substances on the mobility of toxic metals in a mature landfill, Waste Manage. Res., 1999, 17, 296–304.
A. Terbouche, S. Djebbar, O. Benali-Baitich, G. Bouet, Characterization and complexing capacity of humic acid extracted from Yakouren soil with heavy metals by conductimetry and quenching of fluorescence, Soil Sediment Contam., 2010, 19, 21–41.
J. C. G. Esteves da Silva, A. A. S. C. Machado, C. J. S. Oliveira, M. S. S. D. S. Pinto, Fluorescence quenching of anthropogenic fulvic acids by Cu(ii), Fe(iii) and UO22+, Talanta, 1998, 45, 1155–1165.
D. K. Ryan, J. H. Weber, Fluorescence quenching titration for determination of complexing capacities and stability constants of fulvic acid, Anal. Chem., 1982, 54, 986–990.
D. K. Ryan, J. H. Weber, Copper(ii) complexing capacities of natural waters by fluorescence quenching, Environ. Sci. Technol., 1982, 16, 866–872.
IHSS, International Humic Substance Society, http://www.humicsubstances.org
F. S. García Einschlag, KINESIM 9.5, Software for Kinetic and Spectrophotometric Analysis, Argentina, 2005.
J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Kluwer Academic/Plenum Publisher, New York, U.S.A., 2nd edn, 1999, ch. 8, pp. 238–264.
S. S. Leher, Solute perturbation of protein fluorescence. Quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion, Biochemistry, 1971, 10, 3254–3253.
C. Xiaoli, L. Guixiang, Z. Xin, H. Yongxia, Z. Youcai, Complexion between mercury and humic substances from different landfill stabilization processes and its implication for the environment, J. Hazard. Mater., 2012, 209–210, 59–66.
Y. C. Bai, F. C. Wu, C. Q. Liua, W. Lia, J. Y. Guoa, P. Q. Fua, B. S. Xing, J. Zheng, Ultraviolet absorbance titration for determining stability constants of humic substances with Cu(ii) and Hg(ii), Anal. Chim. Acta, 2008, 616, 115–121.
Y. P. Chin, G. Alken, E. O’Loughlin, Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances, Environ. Sci. Technol., 1994, 28, 1853–1858.
C. Guéguen, C. W. Cuss, Characterization of aquatic dissolved organic matter by asymmetrical flow field-flow fractionation coupled to UV–Visible diode array and excitation emission matrix fluorescence, J. Chromatogr., A, 2011, 1218, 4188–4198.
A. Brown, D. M. McKnight, Y. P. Chin, E. C. Roberts, M. Uhle, Chemical characterization of dissolved organic material in Pony Lake, a saline coastal pond in Antarctica, Mar. Chem., 2004, 89, 327–337.
R. A. Mignone, M. V. Martin, F. E. Morán Vieyra, V. I. Palazzi, B. López de Mishima, D. O. Mártire, C. D. Borsarelli, Modulation of optical properties of dissolved humic substances by their molecular complexity, Photochem. Photobiol., 2012, 88, 792–800.
D. S. Smith, R. A. Bell, J. R. Kramer, Metal speciation in natural waters with emphasis on reduced sulfur groups as strong metal binding sites, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2002, 133, 65–74.
K. Gårdfeldt, M. Jonsson, Is bimolecular reduction of Hg(ii) complexes possible in aqueous systems of environmental importance, J. Phys. Chem. A, 2003, 107, 4478–4482.
K. J. Powell, P. L. Brown, R. H. Byrne, T. Gajda, G. Hefter, S. Sjöberg, H. Wanner, Chemical speciation of environmentally significant heavy metals with inorganic ligands part 1: The Hg2+, Cl−, OH−, CO32−, SO42−, and PO43− aqueous systems, Pure Appl. Chem., 2005, 77, 739–800.
W. Stumm and J. J. Morgan, Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, Wiley, New York, U.S.A., 3rd edn, 1996.
U. Skyllberg, K. Xia, P. R. Bloom, E. A. Nater, W. F. Bleam, Binding of mercury(ii) to reduced sulfur in soil organic matter along upland-peat soil transects, J. Environ. Qual., 2000, 29, 855–865.
W. Dong, Y. Bian, L. Y. Liang, B. Gu, Binding constants of mercury and dissolved organic matter determined by a modified ion exchange technique, Environ. Sci. Technol., 2011, 45, 3576–3583.
L. D. Pettit and K. J. Powell, IUPAC Stability Constants Database, version 4.11, Academic Software, Yorks, U.K., 1999.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/c2pp25280e
Rights and permissions
About this article
Cite this article
Berkovic, A.M., García Einschlag, F.S., Gonzalez, M.C. et al. Evaluation of the Hg2+ binding potential of fulvic acids from fluorescence excitation—emission matrices. Photochem Photobiol Sci 12, 384–392 (2013). https://doi.org/10.1039/c2pp25280e
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c2pp25280e