Heterogeneous reactivity of NO and HNO3 on mineral dust in the presence of ozone
Abstract
The co-adsorption and reaction of NO and O3 on authentic Saharan dust surfaces was investigated in a Knudsen reactor at ≈296 K. NO was consumed on the dust surface in the presence of O3 and thereby partially converted to NO2, which was released to the gas-phase. An empirical expression for the dependence of NO2 production, PNO2, on the O3 and NO concentrations and dust surface area could be formulated, allowing the importance of this process in the atmosphere to be assessed:
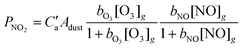 , where Adust is the dust surface area per cubic centimetre of air, C′a = 1.4 × 109 s−1 cm−2, bO3 = 4.0 × 10−12 cm3, and bNO = 5.4 × 10−13 cm3. Even at high tropospheric dust concentrations, this production rate is too low to significantly supplement that from NO oxidation by O3 and HO2. Experiments to examine chemical ageing of dust by ozone showed that HNO3 uptake was not influenced by the presence of 100 ppb O3
(STP), and HNO3 uptake coefficients were similar to those previously derived.
, where Adust is the dust surface area per cubic centimetre of air, C′a = 1.4 × 109 s−1 cm−2, bO3 = 4.0 × 10−12 cm3, and bNO = 5.4 × 10−13 cm3. Even at high tropospheric dust concentrations, this production rate is too low to significantly supplement that from NO oxidation by O3 and HO2. Experiments to examine chemical ageing of dust by ozone showed that HNO3 uptake was not influenced by the presence of 100 ppb O3
(STP), and HNO3 uptake coefficients were similar to those previously derived.

 Please wait while we load your content...
Please wait while we load your content...