A DFT study to unravel the ligand exchange kinetics and thermodynamics of OsVIII oxo/hydroxido/aqua complexes in aqueous matrices†
Abstract
The OsVIII oxo/hydroxido complexes that are abundant in mild to relatively concentrated basic aqueous solutions are OsVIIIO4, [OsVIIIO4(OH)]− and two cis-[OsVIIIO4(OH)2]2− species. OsVIII complexes that contain water ligands are thermodynamically unfavoured w.r.t. the abovementioned species. OsVIIIO4 reacts with hydroxide in two, consecutive, elementary coordination sphere expansion steps to form the [OsVIIIO4(OH)]− complex and then the cis-[OsVIIIO4(OH)2]2− species. The Gibbs energy of activation for both reactions, in the forward and reverse direction, are in the range of 6–12 kcal mol−1 and are relatively close to diffusion-controlled. The thermodynamic driving force of the first reaction is the bonding energy of the OsVIII–OH metal-hydroxido ligand, while of the second reaction it is the relatively large hydration energy of the doubly-charged cis-[OsVIIIO4(OH)2]2− product compared to the singly-charged reactants. The DFT-calculated (PBE-D3 functional) 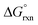 in the simulated aqueous phase (COSMO) is −2.4 kcal mol−1 for the first reaction and −0.6 kcal mol−1 for the second reaction and agree to within 1 kcal mol−1 with reported experimental values, at −2.7 and 0.3 kcal mol−1 respectively. From QTAIM and EDA analyses it is deduced that the OsVIII
in the simulated aqueous phase (COSMO) is −2.4 kcal mol−1 for the first reaction and −0.6 kcal mol−1 for the second reaction and agree to within 1 kcal mol−1 with reported experimental values, at −2.7 and 0.3 kcal mol−1 respectively. From QTAIM and EDA analyses it is deduced that the OsVIII![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonding interactions are ionic (closed-shell) and that OsVIII–OH bonding interactions are polar covalent (dative). In contrast to QTAIM, NCI analysis allowed for the identification of relatively weak intramolecular hydrogen bonding interactions between neighbouring oxo and hydroxido ligands in both [OsVIIIO4(OH)]− and cis-[OsVIIIO4(OH)2]2− complexes.
O bonding interactions are ionic (closed-shell) and that OsVIII–OH bonding interactions are polar covalent (dative). In contrast to QTAIM, NCI analysis allowed for the identification of relatively weak intramolecular hydrogen bonding interactions between neighbouring oxo and hydroxido ligands in both [OsVIIIO4(OH)]− and cis-[OsVIIIO4(OH)2]2− complexes.


 Please wait while we load your content...
Please wait while we load your content...