Abstract
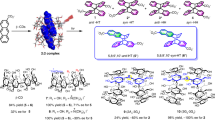
Wavelength effects on the enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate (AC) mediated by native and modified γ-cyclodextrins (CDs) were examined in different solvents at varying temperatures to manipulate the photochirogenic outcomes beyond the thermodynamically determined re/ si-enantiotopic face selectivity upon 2:1 complexation of AC with CD in the ground state. Indeed, the stereochemical outcomes, i.e. syn/ anti, head-to-tail/ head-to-head (HT/HH) and in particular enantiomer ratios, were critical functions of the irradiation wavelength, irrespective of the CD host employed. Furthermore, the wavelength effects observed strongly depended on the host structure, reaction temperature and solvent employed, for which altered stacking geometry of the complexed AC pair is thought to be responsible. By optimizing the irradiation wavelength, chiral host, temperature and solvent, an enantiomeric excess of up to 54 and −37% were achieved for chiral syn-HT and anti-HH dimers.
Similar content being viewed by others
Notes and references
G. S. Hammond and R. S. Cole, Asymmetric induction during energy transfer, J. Am. Chem. Soc. 1965, 87, 3256–3257.
C. Mueller, A. Bauer and T. Bach, Light-driven enantioselective organocatalysis, Angew. Chem., Int. Ed. 2009, 48, 6640–6642.
Y. Inoue, Asymmetric photochemical reactions in solution, Chem. Rev. 1992, 92, 741–770.
A. G. Griesbeck and J. Mattay, Synthetic Organic Photochemistry, CRC Press, 2004.
A. G. Griesbeck and U. J. Meierhenrich, Asymmetric photochemistry and photochirogenesis, Angew. Chem., Int. Ed. 2002, 41, 3147–3154.
C. Yang, Recent progress in supramolecular chiral photochemistry, Chin. Chem. Lett. 2013, 24, 437–441.
M. Nishijima, T. Wada, T. Mori, T. C. S. Pace, C. Bohne and Y. Inoue, Highly enantiomeric supramolecular [4+4] photocyclodimerization of 2-anthracenecarboxylate mediated by human serum albumin, J. Am. Chem. Soc. 2007, 129, 3478–3479.
T. Bach, H. Bergmann, B. Grosch and K. Harms, Highly enantioselective intra- and intermolecular [2+2] photocycloaddition reactions of 2-quinolones mediated by a chiral lactam host: host-guest interactions, product configuration, and the origin of the stereoselectivity in solution, J. Am. Chem. Soc. 2002, 124, 7982–7983.
K. C. W. Chong, J. Sivaguru, T. Shichi, Y. Yoshimi, V. Ramamurthy and J. R. Scheffer, Use of chirally modified zeolites and crystals in photochemical asymmetric synthesis, J. Am. Chem. Soc. 2002, 124, 2858–2859.
N. J. Turro, V. Ramamurthy, W. Cherry and W. Farneth, The effect of wavelength on organic photoreactions in solution. Reactions from upper excited states, Chem. Rev. 1977, 77, 125–145.
Chiral photochemistry, ed. Y. Inoue and V. Ramamurthy, Marcel Dekker, 2004.
C. Yang and Y. Inoue, Supramolecular photochirogenesis, in Supramolecular Photochemistry: Controlling Photochemical Processes, ed. V. Ramamurthy and Y. Inoue, John Wiley & Sons, Inc., Hoboken, 2011, pp. 115–153.
Y. Inoue, H. Ikeda, M. Kaneda, T. Sumimura, S. R. L. Everitt and T. Wada, J. Am. Chem. Soc. 2000, 122, 406–407.
Y. Inoue, Entropy-controlled chemistry. Approach from chiral photochemistry, Kagaku to Kogyo 2006, 59, 152–154.
G. Fukuhara, T. Mori, T. Wada and Y. Inoue, Entropy-controlled supramolecular photochirogenesis: enantiodifferentiating Z–E photoisomerization of cyclooctene included and sensitized by permethylated 6- O-modified β-cyclodextrins, J. Org. Chem. 2006, 71, 8233–8243.
G. Fukuhara, T. Mori and Y. Inoue, Competitive enantiodifferentiating anti-Markovnikov photoaddition of water and methanol to 1,1-diphenylpropene using a sensitizing cyclodextrin host, J. Org. Chem. 2009, 74, 6714–6727.
Y. Inoue, N. Yamasaki, T. Yokoyama and A. Tai, Highly enantiodifferentiating photoisomerization of cyclooctene by congested and/or triplex-forming chiral sensitizers, J. Org. Chem. 1993, 58, 1011–1018.
G. Fukuhara, T. Mori, T. Wada and Y. Inoue, Entropy-controlled supramolecular photochirogenesis: enantiodifferentiating Z–E photoisomerization of cyclooctene included and sensitized by permethylated 6- O-benzoyl-β-cyclodextrin, Chem. Commun. 2005 4199–4200.
A. Nakamura and Y. Inoue, Supramolecular catalysis of the enantiodifferentiating [4+4] photocyclodimerization of 2-anthracenecarboxylate by γ-cyclodextrin, J. Am. Chem. Soc. 2003, 125, 966–974.
T. Wada, M. Nishijima, T. Fujisawa, N. Sugahara, T. Mori, A. Nakamura and Y. Inoue, Bovine serum albumin-mediated enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate, J. Am. Chem. Soc. 2003, 125, 7492–7493.
A. Nakamura and Y. Inoue, Electrostatic manipulation of enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate within γ-cyclodextrin cavity through chemical modification. Inverted product distribution and enhanced enantioselectivity, J. Am. Chem. Soc. 2005, 127, 5338–5339.
C. Yang, G. Fukuhara, A. Nakamura, Y. Origane, K. Fujita, D.-Q. Yuan, T. Mori, T. Wada and Y. Inoue, Enantiodifferentiating [4+4] photocyclodimerization of 2-anthracenecarboxylate catalyzed by 6A,6X-diamino-6A,6X-dideoxy-γ-cyclodextrins: manipulation of product chirality by electrostatic interaction, temperature and solvent in supramolecular photochirogenesis, J. Photochem. Photobiol., A 2005, 173, 375–383.
C. Yang, A. Nakamura, G. Fukuhara, Y. Origane, T. Mori, T. Wada and Y. Inoue, Pressure and temperature-controlled enantiodifferentiating [4+4]-photocyclodimerization of 2-anthracenecarboxylate mediated by secondary face- and skeleton-modified γ-cyclodextrins, J. Org. Chem. 2006, 71, 3126–3136.
C. Yang, A. Nakamura, T. Wada and Y. Inoue, Enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid mediated by γ-cyclodextrins with a flexible or rigid cap, Org. Lett. 2006, 8, 3005–3008.
C. Yang, M. Nishijima, A. Nakamura, T. Mori, T. Wada and Y. Inoue, A remarkable stereoselectivity switching upon solid-state versus solution-phase enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid mediated by native and 3,6-anhydro-γ-cyclodextrins, Tetrahedron Lett. 2007, 48, 4357–4360.
C. Yang, T. Mori, Y. Origane, Y. H. Ko, N. Selvapalam, K. Kim and Y. Inoue, Highly stereoselective photocyclodimerization of α-cyclodextrin-appended anthracene mediated by γ-cyclodextrin and cucurbit[8]uril: a dramatic steric effect operating outside the binding site, J. Am. Chem. Soc. 2008, 130, 8574–8575.
C. Ke, C. Yang, T. Mori, T. Wada, Y. Liu and Y. Inoue, Enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid mediated by a non-sensitizing chiral metallosupramolecular host, Angew. Chem., Int. Ed. 2009, 48, 6675–6678.
H. Qiu, C. Yang, Y. Inoue and S. Che, Supramolecular photochirogenesis with cyclodextrin-silica composite. enantiodifferentiating photocyclodimerization of 2-anthrancenecarboxylate with mesoporous silica wall-capped γ-cyclodextrin, Org. Lett. 2009, 11, 1793–1796.
A. Dawn, N. Fujita, S. Haraguchi, K. Sada and S. Shinkai, An organogel system can control the stereochemical course of anthracene photodimerization, Chem. Commun. 2009 2100–2102.
Y. Ishida, Y. Kai, S. Kato, A. Misawa, S. Amano, Y. Matsuoka and K. Saigo, Theoretical and experimental investigations of circular dichroism and absolute configuration determination of chiral anthracene photodimers, Angew. Chem. 2008, 120, 8365–8369.
A. Wakai, H. Fukasawa, C. Yang, T. Mori and Y. Inoue, Theoretical and experimental investigations of circular dichroism and absolute configuration determination of chiral anthracene photodimers, J. Am. Chem. Soc. 2012, 134, 4990–4997.
Q. Wang, C. Yang, C. Ke, G. Fukuhara, T. Mori, Y. Liu and Y. Inoue, Wavelength-controlled supramolecular photocyclodimerization of anthracenecarboxylate mediated by γ-cyclodextrins, Chem. Commun. 2011, 47, 6849–6851.
M. Nishijima, T. Wada, K. Nagamori and Y. Inoue, High-sensitivity HPLC quantification of nonfluorescent but photolabile analyte through photoreversion in fluorescence detector, Chem. Lett. 2009 726–727.
T. Tamaki, T. Kokubu and K. Ichimura, Regio and stereoselective photodimerization of anthracene derivatives included by cyclodextrins, Tetrahedron 1987, 43, 1485–1488.
T. Tamaki and T. Kokubu, Acceleration of the photodimerization of water-soluble anthracenes included by β- and γ-cyclodextrins, J. Inclusion Phenom. 1984, 2, 815–822.
C. Yang, T. Mori and Y. Inoue, Supramolecular enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate mediated by capped γ-cyclodextrins: Critical control of enantioselectivity by cap rigidity, J. Org. Chem. 2008, 73, 5786–5794.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This paper is dedicated to the memory of Prof. Nicholas Turro.
Rights and permissions
About this article
Cite this article
Yang, C., Wang, Q., Yamauchi, M. et al. Manipulating γ-cyclodextrin-mediated photocyclodimerization of anthracenecarboxylate by wavelength, temperature, solvent and host. Photochem Photobiol Sci 13, 190–198 (2014). https://doi.org/10.1039/c3pp50255d
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c3pp50255d