Abstract
Adenylyl cyclase 3 (Adcy3), a member of the mammalian adenylyl cyclase family responsible for generating the second messenger cAMP, has long been known to play an essential role in olfactory signal transduction. Here, we demonstrated that Adcy3 heterozygous null mice displayed increased visceral adiposity in the absence of hyperphagia and developed abnormal metabolic features characterized by impaired insulin sensitivity, dyslipidemia and increased plasma levels of proinflammatory cytokines on both chow and high-fat diet (HFD). Of note, HFD decreased the Adcy3 expression in white adipose tissue, liver and muscle. We also report for the first time that Adcy3 haploinsufficiency resulted in reduced expression of genes involved in thermogenesis, fatty acid oxidation and insulin signaling, with enhanced expression of genes related to adipogenesis in peripheral tissues of mice. In conclusion, these findings suggest that cAMP signals generated by Adcy3 in peripheral tissues may play a pivotal role in modulating obesity and insulin sensitivity.
Similar content being viewed by others
Introduction
Adenylyl cyclases (Adcys), the downstream enzymes for G-protein-coupled receptors (GPCRs), catalyze the conversion of adenosine triphosphate (ATP) into the universal second messenger cyclic AMP (cAMP) which mediates several physiological functions in mammals including embryogenesis, hormone secretion, glycogen breakdown, smooth-muscle relaxation, cardiac contraction and olfaction1,2,3. Nine isoforms of membrane-bound Adcys are known, each encoded by a distinct gene. Depending on the properties and the relative levels of the isoforms expressed in a tissue or a cell type at a specific time, extracellular signals received through GPCRs can be integrated differently. In the mammalian brain, mRNA for Adcy1 and Adcy2 is highly expressed in regions associated with learning and memory, including the hippocampus, cerebral cortex and cerebellum1, while primary cilia of bile cholangiocytes4, bone cells5 and renal epithelial cells6 are known to express Adcy3, Adcy4, Adcy6 and Adcy8.
Adcy3 is a 130-kDa glycosylated protein involved in the cascade required for detection of odorants in olfactory neurons7. Coupling of odorant receptors to Adcy3 stimulates cAMP transients that function as the major second messengers for olfactory signaling7. Adcy3 knockout mice (Adcy3−/−) are known to be anosmic (unable to smell) and to have a very high fatality rate after birth. It is believed that the neonatal mortality is due to the inability of pups to smell their mother, compromising their ability to nurse8. Besides the well-known function of Adcy3 in regulating olfaction, it has also been suggested to exert ectopic functions based on studies of Adcy3-deficient mice9,10,11. Inactivation of the Adcy3 gene significantly reduced male fertility: spermatozoa from Adcy3−/− male mice exhibited decreased motility and showed an increase in spontaneous acrosome reactions9,10. Moreover, plasma renin and glomerular filtration rates were significantly decreased in Adcy3−/− mice, implying that Adcy3 may play a critical role in regulating fundamental aspects of renal function11.
Several lines of evidence suggest the interesting possibility that Adcy3 may play an important role in the regulation of adiposity. For example, genetic association studies of the Adcy3 in Swedish12 and Chinese13 populations led to the discovery of Adcy3 polymorphisms associated with decreased risk of obesity. Furthermore, Wang et al. reported that Adcy3−/− mice fed chow exhibited higher total fat mass measured by whole body composition analyzer, hyperphagia and lower physical activity and speculated that these phenotypic changes conferred by Adcy3 knockout were associated with disruption of cAMP signaling in primary cilia of the hypothalamus14. Pitman et al. recently found that the gain-of-function mutation of Adcy3 gene had increased Adcy3 activity and cAMP production and consequently the mutant mice had significantly lower total body weights and fat mass compared to wild type controls after 12 weeks of HFD feeding15. It has been established that cAMP signaling, in addition to its involvement in regulating feeding behavior and leptin sensitivity in the hypothalamus16,17, also has a role in controlling adipose tissue development and function through regulating the expressions of genes related to adipogenesis, lipolysis and thermogenesis18,19,20. There is also some evidence to suggest Adcy3 may play physiological roles in muscle and liver. For example, Griffin et al. found that Adcy3 is expressed in primary muscle cell and is associated with muscle cell migration and adhesion21. In addition, hepatic Adcy3 was reported to play a protective role in insulin resistance and obesity in mice with HFD-induced obesity22. The present study was aimed at determining whether Adcy3 functions in peripheral tissues related to metabolic disorders by regulating the development of adiposity and insulin resistance in mice.
Results
Adcy3+/− mice display increased visceral adiposity
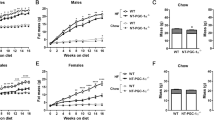
Adcy3+/− mice maintained on HFD for 17 weeks exhibited a significant increase in final body weight (14% for male, p < 0.05; 26% for female, p < 0.01), cumulative body weight gain (20% for male, p < 0.05; 63% for female, p < 0.05) and total visceral fat-pad weights (27% for male, p < 0.05; 133% for female, p < 0.01) as compared to WT mice (Fig. 1a,b,e–g,j). There was no significant difference in food intake between Adcy3+/− and WT mice for male and female (Fig. 1c,h). The food efficiency ratio in Adcy3+/− mice was significantly higher (16% for male, p < 0.05; 100% for female, p < 0.05) as compared to WT mice under conditions of HFD feeding (Fig. 1d,i). Male Adcy3+/− mice fed chow were found to have significantly higher visceral fat-pad weight as compared to WT mice (Fig. 1e).
Adcy3+/− mice are prone to develop visceral adiposity.
(a,f) Changes in body weight during 17 weeks of feeding. (b,g) Cumulative body weight gain. (c,h) Daily food intake. (d,i) Food efficiency ratio (FER; bodyweight gain over the experimental period [g]/food intake over the experimental period [g]). (e,j) Visceral fat-pad weight. Data for both male (upper panels) and female (lower panels) mice are presented. Values are presented as means ± SEM (n = 8). Significant differences between groups are indicated by asterisks; *P < 0.05; **P < 0.01; ***P < 0.001.
Plasma and hepatic biochemical parameters in mice
Adcy3+/− mice on HFD exhibited significantly higher levels of plasma TG (29% for male, p < 0.05; 26% for female, p < 0.05), TC (20% for male, p < 0.05; 23% for female, p < 0.05) and FFA (27% for male, p < 0.05; 26% for female) relative to WT mice (Fig. 2a–c,j–l). Hepatic triglyceride (55% for male, p < 0.001; 60% for female, p < 0.001), cholesterol (77% for male, p < 0.001; 79% for female, p < 0.001) and fatty acid (216% for male, p < 0.001; 234% for female, p < 0.001) levels were significantly higher in Adcy3+/− mice relative to levels observed in WT mice on HFD (Fig. 2d–f,m–o). Adcy3+/− mice had significantly higher plasma concentrations of leptin (10% for male, p < 0.05; 19% for female, p < 0.05), IL-6 (16% for male, p < 0.05; 23% for female, p < 0.05) and TNFα (10% for male, p < 0.05; 12% for female, p < 0.05) as compared to WT mice on HFD (Fig. 2g–i,p–r). Adcy3+/− mice fed chow had significantly higher hepatic triglyceride (23% for male, p < 0.01; 25% for female, p < 0.01), cholesterol (28% for male, p < 0.01; 32% for female, p < 0.01) and fatty acid (96% for male, p < 0.01; 128% for female, p < 0.01) levels than WT mice (Fig. 2d–f,m–o). In the meantime, there was no significant difference in plasma levels of TG, TC, FFA and proinflammatory cytokines between Adcy3+/− and WT mice fed chow irrespective of gender (Fig. 2a–c,g–i,j–l,p–r).
Plasma and hepatic biochemical parameters in mice.
Plasma levels of (a,j) triglyceride, (b,k) total cholesterol and (c,l) FFA. Hepatic contents of (d,m) triglyceride, (e,n) cholesterol and (f,o) FFA. Plasma levels of (g,p) leptin, (h,q) IL-6 and (i,r) TNFα. Data for both male (upper panels) and female (lower panels) mice are presented. Values are presented as mean ± SEM (n = 8). Significant differences between groups are indicated by asterisks; *P < 0.05; **P < 0.01; ***P < 0.001.
Adcy3+/− mice display impaired glucose homeostasis
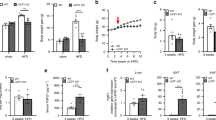
The oral glucose tolerance test (OGTT) was performed 2 weeks prior to the end of the experimental period. Integrated plasma glucose concentration, as calculated by area under the curve (AUC), was increased in Adcy3+/− mice relative to their WT counterparts on both chow (18% for male, p < 0.05; 13% for female) and HFD (26% for male, p < 0.05; 32% for female, p < 0.05; Fig. 3a,b,f,g). Fasting plasma glucose and insulin levels were measured at the end of the feeding period. Adcy3+/− mice fed HFD had significantly higher fasting plasma concentrations of glucose (42% for male, p < 0.05; 69% for female, p < 0.05; Fig. 3c,h) and insulin (28% for male, p < 0.05; 38% for female, p < 0.05; Fig. 3d,i) compared with WT mice. Along with the plasma glucose and insulin levels, the HOMA-IR values indicated that insulin sensitivity was significantly decreased in Adcy3+/− mice maintained on HFD (Fig. 3e,j).
Adcy3+/− mice have impaired glucose tolerance and are less insulin sensitive.
(a,f) Oral glucose tolerance test. (b,g) Area under the curve. (c,h) Fasting plasma glucose levels. (d,i) Fasting insulin levels. (e,j) Homeostasis model assessment of basal insulin resistance. Data for both male (upper panels) and female (lower panels) mice are presented. Values are presented as means ± SEM (n = 8). Significant differences between groups are indicated by asterisks; *P < 0.05; **P < 0.01; ***P < 0.001.
The protein levels of phosphor-IR, phosphor-IRS1, phosphor-AKT, phosphor-GSK3β were significantly decreased in the epididymal adipose tissue of Adcy3+/− mice relative to WT mice on both chow and HFD (Fig. 4a). The amount of GLUT4 found in the membrane fraction of the epididymal adipose tissue was significantly decreased, whereas the amount of total cellular GLUT4 remained the same in Adcy3+/− mice when compared with WT mice (Fig. 4b). Similarly, the protein levels of phosphor-IRS1 and phosphor-AKT were significantly decreased in the liver and muscle of Adcy3+/− mice relative to WT mice on both chow and HFD (Fig. 4c,d). The expression of the key gluconeogenesis enzymes, such as PEPCK and G6pase, were found to be significantly increased in the liver of Adcy3+/− mice than in WT mice on both chow and HFD (Fig. 4e).
Adcy3 haploinsufficiency led to impairment of insulin signaling.
(a,b) Protein levels of p-IR, IR, p-IRS1, IRS1, p-AKT, AKT, p-GSK3β, GSK3β, membrane GLUT4 and total GLUT4 in the epididymal adipose tissue. (c,d) Protein levels of p-IRS1, IRS1, p-AKT and AKT in the (c) liver and (d) muscle. (e) mRNA levels of G6Pase and PEPCK in the liver of mice. (f) The knockdown efficiency by siRNA was monitored by semiquantitative RT-PCR. (g) Insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes treated with control siRNA or siRNA against Adcy3. The full-length blots/gels are presented in Supplementary Figs 1–7. Values are presented as means ± SEM (n = 8). Significant differences between groups are indicated by asterisks; *P < 0.05; **P < 0.01; ***P < 0.001.
Insulin-stimulated GLUT4 translocation is decreased in Adcy3 deficient 3T3-L1 adipocytes
To test whether Adcy3 affects the insulin-stimulated GLUT4 translocation from cytosol to plasma membrane, we transiently knocked down Adcy3 using siRNA in 3T3-L1 adipocytes and measured the membrane-bound GLUT4 in the presence of insulin by western blotting. Relative to scrambled siRNA-transfected controls, treatment with siRNA (Adcy3 SMARTpool) for 48 h achieved significant reductions of Adcy3 expression level in 3T3-L1 adipocytes (Fig. 4f). The loss of Adcy3 led to a significant decrease in the amount of plasma membrane GLUT4 after insulin stimulation (Fig. 4g).
Adcy3 expression in peripheral tissues of mice
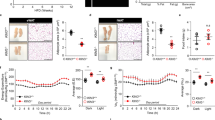
HFD feeding significantly downregulated the mRNA expression of Adcy1, Adcy2, Adcy3 and Adcy8 in the epididymal adipose tissue of mice but had no effect on the expression of other Adcy isoforms (Fig. 5e). Adcy3+/− mice showed significantly decreased total Adcy activity in the epididymal adipose tissue as compared to WT mice fed chow (−31%, p < 0.01) and HFD (−16%, p < 0.01) (Fig. 5a). Both mRNA and protein levels of Adcy3 were significantly reduced in the epididymal adipose tissue of Adcy3+/− mice than in WT mice irrespective of diet (Fig. 5b,e). The protein level of Adcy3 was also significantly decreased in the liver and muscle of Adcy3+/− mice relative to WT mice on both chow and HFD. (Fig. 5c,d).
Adcy3 expression in peripheral tissues of mice.
(a) cAMP concentrations in the epididymal adipose tissue of mice. (b–d) Protein level of Adcy3 in the (b) epididymal adipose tissue, (c) liver and (d) muscle of mice. (e) mRNA levels of Adcy isoforms in the epididymal adipose tissue of mice. The full-length blots/gels are presented in Supplementary Figs 8–11. Values are presented as means ± SEM (n = 8). Significant differences between groups are indicated by asterisks; *P < 0.05; **P < 0.01; ***P < 0.001.
Altered expression of molecules related to adipogenesis, fatty acid oxidation and thermogenesis in Adcy3+/− mice
The protein levels of PKA, phosphor-AMPK, phosphor-HSL and phosphor-CREB were found to be significantly lower in the epididymal adipose tissue of Adcy3+/− mice than in WT mice on both chow and HFD (Fig. 6b–d). The expression levels of PKA and phosphor-AMPK were also significantly decreased in the liver and muscle of Adcy3+/− mice compared with WT mice irrespective of diet (Fig. 6e,f). The expression of genes encoding transcription factors, such as peroxisome proliferator-activated receptor γ2 (PPARγ2) and CCAAT/enhancer binding protein α (C/EBPα) and their targets, adipocyte fatty acid binding protein (aP2) and fatty acid synthase (FAS), were significantly increased in the epididymal adipose tissue of Adcy3+/− mice relative to WT mice on both chow and HFD (Fig. 6a). The mRNA level of carnitine palmitoyl transferase 1 (CPT1) were significantly downregulated by Adcy3 haploinsufficiency in the epididymal adipose tissue of mice on both chow and HFD (Fig. 6h). Adcy3 haploinsufficiency significantly decreased the expression of thermogenic genes, such as peroxisome proliferator-activated receptor gamma co-activator 1-alpha (PGC1α), encoding PR-domain containing 16 (PRDM16), uncoupling protein 1 (UCP1), T-box transcription factor (TBX1) and transmembrane protein 26 (TMEM26) in both epididymal and subcutaneous adipose tissues (Fig. 6g,i).
Adcy3+/− mice display altered expression of genes related to adipogenesis, fatty acid oxidation and thermogenesis.
(a) mRNA levels of PPARγ2, C/EBPα and their target genes in the epididymal adipose tissues of mice. (b) Protein levels of p-AMPK and total AMPK in the epididymal adipose tissues of mice. (c) Protein levels of PKA, p-HSL and total HSL in the epididymal adipose tissues of mice. (d) Protein levels of p-CREB and total CREB in the epididymal adipose tissues of mice. (e,f) Protein levels of PKA and p-AMPK in the (e) liver and (f) muscle of mice. (g,h) mRNA levels of thermogenic genes in the (g) epididymal and (h) subcutaneous adipose tissues of mice. (i) Gene expression of CPT1 in the epididymal adipose tissue of mice. The full-length blots/gels are presented in Supplementary Figs 12–20. Values are presented as means ± SEM (n = 8). Significant differences between groups are indicated by asterisks; *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Adcy3+/− mice (Adcy3tm1Dgen/J) on a 129/OlaHsd background used in the present study were offspring from heterozygous intercross matings of mice originally obtained from the Jackson Laboratory and were found to be healthy and viable. In contrast, homozygous Adcy3 mice on a 129/OlaHsd background showed a postnatal lethal phenotype23 and other homozygous Adcy3 mice on a 129Sv/J background (Adcy3tm1Drs) struggled to survive, with an 80% fatality rate within 48 hours and exhibited abnormal phenotypes, such as anosmia8, reduced male fertility10 and excretory dysfunction of kidney11. In the present study, the increased weight gain and adiposity observed in the Adcy3+/− mice were not associated with the increased consumption of food, thereby suggesting that Adcy3 haploinsufficiency does not promote hyperphagia. Consistent with our findings, Pitman et al. recently revealed that mice carrying a gain-of-function mutation in Adcy3 (Adcy3Jll/+) were protected from diet-induced obesity due to increased oxygen consumption and physical activity without a change in food intake compared to their WT littermates during the 7 days of whole-body metabolic measurements15.
In the present study, all membrane-bound Adcy isoforms reported, from Adcy1 through Adcy9, were expressed in white adipose tissue (WAT) of mice (Fig. 5). Nevertheless, Adcy3 appears to be one of the major isoforms contributing to Adcy activity in WAT based on our observation that Adcy3 haploinsufficiency resulted in a significant decrease (–31%) in total Adcy activity in visceral adipose tissue of mice on chow. Chaudhry et al. reported that in brown adipose tissue of rats, the increase in Adcy activity corresponded to a selective upregulation of Adcy3 expression during the neonatal period when offspring are especially sensitive to environmental conditions and maintenance of body temperature24. Moreover, treatment of neonates with the sympathetic neurotoxin 6-hydroxydopamine abolished the perinatal increase in both Adcy activity and Adcy3 mRNA levels without affecting the expression of other Adcy isoforms24.
In the present study, the HFD-induced decrease in total Adcy activity observed in visceral adipose tissue of both WT and Adcy3+/− mice was accompanied by downregulation of Adcy1, Adcy2, Adcy3 and Adcy8 mRNA expression. This finding is in line with previous reports indicating that expression of Adcy isoforms in a specific cell or tissue type can be selectively changed in response to pathophysiologic stimuli25,26. Choi et al. observed that treatment of human colon epithelial cells with butyrate, which induced cell differentiation, downregulated mRNA expression of Adcy3, Adcy4, Adcy6, and Adcy7 by 70–90%, while mRNA levels of the other three isoforms, including Adcy1, Adcy5 and Adcy9, were unchanged25. Furthermore, Suzuki et al. reported that among four isoforms of Adcys (Adcy2, Adcy6, Adcy7 and Adcy9) detected in the gastrocnemius muscle, Adcy2 and Adcy9 mRNA were selectively downregulated after denervation carried out by excision of the left sciatic nerve at the midthigh region26.
The cAMP signaling pathways are pivotal in regulation of adipose tissue development and function27. The accumulation of cAMP activates PKA in adipocytes of all colors and origins, thereby phosphorylating several proteins, such as CREB20, AMPK28 and HSL29 (Fig. 7). Phosphor-CREB then activates the expression of PGC-1α, which induces the transcription of downstream thermogenic genes, including UCP120. In parallel, phosphorylated AMPK inhibits preadipocyte differentiation by downregulating PPARγ and C/EBPα, which are the central regulators of adipogenesis and lipid storage in adipocytes30. Moreover, PKA-mediated phosphorylation induces translocation of the HSL to the surface of lipid droplets and enhances its catalytic activity29. The released free fatty acids are oxidized to fatty acyl-CoA which combines with CPT1 to enter mitochondria for its oxidation. In the present study, Adcy3 haploinsufficiency significantly decreased the protein levels of PKA, phosphor-CREB, phosphor-HSL and phosphor-AMPK in WAT of mice fed HFD (Fig. 7). These results indicated that in WAT, Adcy3 might be associated with the regulation of cAMP-PKA-mediated signaling pathways that affect thermogenesis, fatty acid oxidation and adipogenesis.
Here, Adcy3 haploinsufficiency reduced insulin sensitivity in mice, as demonstrated by as demonstrated by impairment of insulin signaling in WAT, liver and muscle, along with increased AUC values and elevated fasting plasma glucose and insulin levels. This insulin resistance observed in Adcy3+/− mice appears to be associated with decreased AMPK activity (Fig. 7). Reduced AMPK activity is known to decrease the phosphorylation of IRS-1 at Ser789, thus negatively regulating insulin signaling in adipocytes31. Moreover, Adcy3+/− mice showed higher plasma levels of proinflammatory cytokines, such as IL-6 and TNFα, relative to their WT littermates on both chow and HFD. It is well established that within adipose tissue, IL-6 and TNFα cause adipocyte insulin resistance through inactivation of both the insulin receptor and IRS-1, both of which result in diminished activation of phosphoinositol-3-kinase, the essential second messenger signal that governs most of metabolic effects associated with insulin32,33. Therefore, the increased plasma levels of proinflammatory cytokines could also be a contributing factor for the aggravated insulin resistance in Adcy3+/− mice.
Possible application of Adcy3 as a target for insulin resistance and anti-obesity treatment has been implicated in a recent study by Liang et al.22 revealing that hepatic Adcy3 is upregulated in mice with HFD-induced obesity by liraglutide, a glucagon-like peptide-1 analogue recently approved by the US Food and Drug Administration as an obesity treatment option. In conclusion, the Adcy3 heterozygous null mice displayed increased visceral adiposity in the absence of hyperphagia and developed abnormal metabolic features on both chow and HFD. We report for the first time that HFD decreased the Adcy3 expression in white adipose tissue, liver and muscle and that Adcy3 haploinsufficiency resulted in reduced expression of genes involved in thermogenesis, fatty acid oxidation and insulin signaling, with enhanced expression of genes related to adipogenesis in peripheral tissues of mice. Although the role of Adcy3 in humans has yet to be elucidated, our findings raise the possibility that Adcy3 activators may be useful agents for the prevention or treatment of obesity and associated metabolic complications.
Methods
Animals and diets
Adcy3+/− mice (B6.129P2-Adcy3tm1Dgen/J; stock 005773) were generated by Deltagen Inc. (San Mateo, CA, USA) and acquired from the Jackson Laboratories (Bar Harbor, ME, Maine, USA) through the “NIH initiative supporting placement of Deltagen, Inc., mice into public repositories.” Heterozygous intercross was performed to produce Adcy3+/− mice and wild-type (WT) littermate control mice. Genotyping was performed using PCR, with genomic DNA extracted from tail tips. Three primers were used: WT (5′-CGT CTT CCT CTA CCT GTG TGC TAT C-3′), mutant (5′-GGG CCA GCT CAT TCC TCC CAC TCA T-3′) and common (5′-TCC TAA CGG ACT TAC ACT GAG GTA G-3′).
Four-week-old male and female Adcy3+/− or WT littermate mice were housed in a pathogen-free facility at 21 ± 2.0 °C, with 50 ± 5% relative humidity and a 12-h light/dark cycle. The mice were provided access to rodent chow and tap water ad libitum for 1 week. Thereafter, the mice (n = 8) were placed on chow or HFD for 17 weeks. The HFD consisted of 200 g fat/kg body weight (170 g of lard and 30 g of corn oil) and 1% (w/w) cholesterol (supplementary Table 1).
Food intake and body weight were measured daily and weekly, respectively. At the end of the experimental period, the mice were anesthetized with diethyl ether after they were fasted for 16 h. Blood samples were drawn from the inferior vena cava into an ethylene-diamine-tetra-acetic acid (EDTA)-coated tube and the plasma samples were obtained by centrifuging the blood at 4,000 g for 15 min at 4 °C. The epididymal, retroperitoneal, mesenteric, perirenal and subcutaneous fat-pads were dissected, removed, weighed and immediately snap-frozen in liquid nitrogen and stored at –80 °C until further use. All animal experiments were performed in accordance with the Korea Food and Drug Administration guidelines. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Yonsei Laboratory Animal Research Center (Permit no. 2011-0062).
Cell culture and transfection
3T3-L1 fibroblasts obtained from American Type Culture Collection (Manassas, VA, USA) were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, penicillin (50 units/mL) and streptomycin (50 mg/mL). The cells were grown at 37 °C in a humidified 5% CO2 atmosphere. We treated confluent cultures with 0.5 mM 3-isobutyl-1-methylxantine, 0.25 mM dexamethasone and 10 mg/mL insulin to promote the differentiation of 3T3-L1 cells into adipocytes. After 2 days, the 3-isobutyl-1-methylxanthine and dexamethasone were removed and insulin was continued for another 2 days. The growth medium was replenished at 2-day intervals until adipocyte differentiation. For Adcy3 knockdown, the differentiated adipocytes were treated with 50 nM of either control siRNA (nontargeting SMARTpool, catalog no. D-001810-10-05, Dharmacon, Lafayette, CO, USA) or siRNA against Adcy3 (On-TARGET plus SMART pool siRNA, catalog no. L-0588903-00-10, Dharmacon) on the ninth day of differentiation. Two days post-transfection, serum–starved cells were treated with 17 nM insulin for 15 min and then lysed for RNA or protein. The knockdown efficiency by siRNA was monitored by semiquantitative RT-PCR.
Biochemical analysis
Plasma content of total cholesterol (TC), triglyceride (TG), free fatty acid (FFA) and glucose were enzymatically determined using individual commercial kits (Bio-Clinical System, Gyeonggi-do, South Korea). Plasma concentration of insulin was measured using a commercially available mouse enzyme-linked immunosorbent assay (ELISA) kit (Millipore, Billerica, MA, USA). The homeostasis model assessment of basal insulin resistance (HOMA-IR) was calculated as [fasting plasma glucose × fasting plasma insulin/22.5] to assess insulin resistance. Hepatic lipids were extracted as described using the method developed by Folch et al.34. Triglyceride, cholesterol and FFA levels in the hepatic lipid extracts were measured using the same enzymatic kits that were used for the plasma analyses.
Oral glucose tolerance test
The OGTT was performed 2 weeks before the end of the experimental period on 6 h-fasted mice by oral glucose administration (gavage with 2 g glucose/kg body weight). Blood glucose was measured from tail blood at times 0, 15, 30, 60, 90 and 120 min after glucose administration.
Western blotting analysis
To obtain total protein, the liver, muscle and epididymal and subcutaneous adipose tissue samples obtained from each mouse were homogenized at 4 °C in an extraction buffer containing 100 mmol/L Tris-HCl (pH 7.4), 5 mmol/L EDTA, 50 mmol/L NaCl, 50 mmol/L sodium pyrophosphate, 50 mmol/L NaF, 100 mmol/L orthovanadate, 1% Triton X-100, 1 mmol/L phenylmethanesulfonyl fluoride, 2 μg/mL aprotinin, 1 μg/mL pepstatin A and 1 μg/mL leupeptin and centrifuged at 13,000 g for 20 min at 4 °C. To obtain membrane protein, the epididymal and subcutaneous adipose tissue samples were homogenized in a buffer containing 20 mmol/L HEPES (pH 7.4), 4 mmol/L EDTA, 250 mmol/L sucrose, two tablets of protein inhibitor, 1 mmol/L sodium orthovanadate and 1% Triton X-100. The homogenates were centrifuged at 2,000 g for 1.5 h at 4 °C and the supernatant fractions were centrifuged at 150,000 g for 1.5 h at 4 °C. Protein concentrations were measured by Bradford assay (Bio-Rad, Hercules, CA, USA).
Total protein or membrane protein (40 μg) was separated by 8% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and then electrophoretically transferred to nitrocellulose membranes (Amersham, Buckinghamshire, UK). The nitrocellulose membranes were incubated overnight with primary antibodies (diluted 1:1,000) at 4 °C. Antibodies to the following proteins were commercially obtained from the indicated sources: β-actin and Adcy3 from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and AMP-activated protein kinase (AMPK), phosphor-AMPK (Thr172), cyclic AMP-responsive element-binding protein (CREB), phosphor-CREB (Ser133), PKA, hormone-sensitive lipase (HSL), phosphor-HSL (Ser563), insulin receptor β subunit (IRβ), phosphor-IRβ (Tyr1162/1163), insulin receptor substrate 1 (IRS1), phosphor-IRS1 (Ser789), protein kinase B (AKT), phosphor-AKT (Ser473), glycogen synthase kinase-3β (GSK3β), phosphor-GSK3β (Ser9) and glucose transporter type 4 (GLUT4) from Cell Signaling Technology (Danvers, MA, USA). Secondary antibodies were applied for 1 hr at room temperature in Tris-buffered saline containing 0.05% Tween-20. Signal was detected by using a chemiluminescent detection system (Amersham). The protein bands were quantified using Quantity One Analysis Software (Bio-Rad).
Adcy enzymatic assay
Fifty microliters of Adcy mixture [100 mM Tris-acetate (pH 7.4), 20 mM KCl, 10 mM MgCl2, 20 mM phosphoenolpyruvate, 100 μg/mg pyruvate kinase, 2 mM ATP, 20 μM guanosine triphosphate, 2 mM dithiothreitol, 0.4 mM bovine serum albumin and 0.1 mM 3-isobutyl-1-methylxanthine] and 125 μg of membrane fractions from epididymal adipose tissue were added into microcentrifugation tubes in duplicate and maintained at 4 °C. The reaction tubes were incubated in a water bath maintained at 37 °C for 30 min and the reaction was terminated by heating at 95 °C for 5 min. The supernatant solution was stored after centrifugation at 10,000 g for 5 min at 4 °C. The cAMP assay was performed using the cAMP ELISA kit from Enzo Life Sciences International, Inc. (Plymouth Metting, PA, USA).
RNA extraction and semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was isolated from the liver, epididymal and subcutaneous adipose tissue of each mouse by using TRIzol (Invitrogen, Carlsbad, CA, USA) and was reverse-transcribed by using the Superscript II Kit (Invitrogen) according to the manufacturer instructions. The PCR was programmed as follows: 10 min at 94 °C, 30–35 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min and 10-min incubation at 72 °C. Then, 5 μL of each PCR product was mixed with 1 μL of 6-fold concentrated loading buffer and electrophoresed on a 2% agarose gel containing ethidium bromide. The band intensities were quantified using the Quantity One Analysis Software (Bio-Rad). The mRNA levels were normalized to that of the glyceraldehyde-3-phosphate dehydrogenase transcript. Sequences of all primers are listed in supplementary Table 2.
Statistics
Results on body weight gain and plasma biochemistries are presented as the mean ± standard error of mean (SEM) of eight mice in each group. The RT-PCR and western blot data are shown as the means ± SEM of three independent experiments (n = 2 or 3 per experiment) for each group, cumulatively including eight mice. An unpaired Student t-test analysis was used for all data comparisons between the Adcy3+/− and WT control mice fed chow or HFD. All statistical analyses were performed with SPSS 21.0 software (IBM, Corp., Armonk, NY, USA) and significance was set at *P < 0.05, **P < 0.01 and ***P < 0.001.
Additional Information
How to cite this article: Tong, T. et al. Adenylyl cyclase 3 haploinsufficiency confers susceptibility to diet-induced obesity and insulin resistance in mice. Sci. Rep. 6, 34179; doi: 10.1038/srep34179 (2016).
References
Hanoune, J. & Defer, N. Regulation and role of adenylyl cyclase isoforms. Annu. Rev. Pharmacol. 41, 145–174 (2001).
Cooper, D. M. Regulation and organization of adenylyl cyclases and cAMP. Biochem. J. 375, 517–529 (2003).
Seed Ahmed, M. et al. Increased expression of adenylyl cyclase 3 in pancreatic islets and central nervous system of diabetic Goto-Kakizaki rats: a possible regulatory role in glucose homeostasis. Islets 4, 343–348 (2012).
Masyuk, A. I. et al. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y(12) purinergic receptors. Am. J. Physiol-Gastr. L. 295, G725–G734 (2008).
Kwon, R. Y., Temiyasathit, S., Tummala, P., Quah, C. C. & Jacobs, C. R. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. Faseb. J. 24, 2859–2868 (2010).
Raychowdhury, M. K. et al. Vasopressin receptor-mediated functional signaling pathway in primary cilia of renal epithelial cells. Am. J. Physiol-Renal 296, F87–F97 (2009).
Wang, H. & Storm, D. R. Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Mol. Pharmacol. 63, 463–468 (2003).
Wong, S. T. et al. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron 27, 487–497 (2000).
Gautier-Courteille, C., Salanova, M. & Conti, M. The olfactory adenylyl cyclase III is expressed in rat germ cells during spermiogenesis. Endocrinology 139, 2588–2599 (1998).
Livera, G. et al. Inactivation of the mouse adenylyl cyclase 3 gene disrupts male fertility and spermatozoon function. Mol. Endocrinol. 19, 1277–1290 (2005).
Pluznick, J. L. et al. Functional expression of the olfactory signaling system in the kidney. Proc. Natl. Acad. Sci. USA 106, 2059–2064 (2009).
Nordman, S. et al. Genetic variation of the adenylyl cyclase 3 (AC3) locus and its influence on type 2 diabetes and obesity susceptibility in Swedish men. Int. J. Obesity 32, 407–412 (2008).
Wang, H. R. et al. Evaluation of the association between the AC3 genetic polymorphisms and obesity in a Chinese Han population. PLoS One 5, e13851 (2010).
Wang, Z. et al. Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS One 4, e6979 (2009).
Pitman, J. L. et al. A gain-of-function mutation in adenylate cyclase 3 protects mice from diet-induced obesity. PLoS One 9, e110226 (2014).
Zhao, A. Z. Control of food intake through regulation of cAMP. Curr. Top. Dev. Biol. 67, 207–224 (2005).
Zhao, A. Z., Huan, J. N., Gupta, S., Pal, R. & Sahu, A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat. Neurosci. 5, 727–728 (2002).
Rogne, M. & Tasken, K. Compartmentalization of cAMP signaling in adipogenesis, lipogenesis and lipolysis. Horm. Metab. Res. 46, 833–840 (2014).
Carmen, G. Y. & Victor, S. M. Signalling mechanisms regulating lipolysis. Cell. Signal. 18, 401–408 (2006).
Cao, W. H. et al. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell. Biol. 24, 3057–3067 (2004).
Griffin, C. A., Kafadar, K. A. & Pavlath, G. K. MOR23 Promotes Muscle Regeneration and Regulates Cell Adhesion and Migration. Dev. Cell 17, 649–661 (2009).
Liang, Y. et al. Hepatic adenylate cyclase 3 is upregulated by Liraglutide and subsequently plays a protective role in insulin resistance and obesity. Nutr. Diabetes 6, e191 (2016).
Deltagen Inc. NIH initiative supporting placement of Deltagen, Inc. mice into public repositories. MGI Direct Data Submission, http://www.informatics.jax.org/reference/j:101679 (2005).
Chaudhry, A., Muffler, L. A., Yao, R. H. & Granneman, J. G. Perinatal expression of adenylyl cyclase subtypes in rat brown adipose tissue. Am. J. Physiol-Reg. I. 270, R755–R760 (1996).
Choi, L. J. et al. Coordinate down-regulation of adenylyl cyclase isoforms and the stimulatory G protein (G(s)) in intestinal epithelial cell differentiation. J. Biol. Chem. 285, 12504–12511 (2010).
Suzuki, Y. et al. Expression of adenylyl cyclase mRNAs in the denervated and in the developing mouse skeletal muscle. Am. J. Physiol-Cell Ph. 274, C1674–C1685 (1998).
Madsen, L. & Kristiansen, K. The importance of dietary modulation of cAMP and insulin signaling in adipose tissue and the development of obesity. Ann. N. Y. Acad. Sci. 1190, 1–14 (2010).
Yin, W., Mu, J. & Birnbaum, M. J. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. J. Biol. Chem. 278, 43074–43080 (2003).
Djouder, N. et al. PKA phosphorylates and inactivates AMPK alpha to promote efficient lipolysis. Embo. J. 29, 469–481 (2010).
Habinowski, S. A. & Witters, L. A. The effects of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem. Bioph. Res. Co. 286, 852–856 (2001).
Liu, Q. Q., Gauthier, M. S., Sun, L., Ruderman, N. & Lodish, H. Activation of AMP-activated protein kinase signaling pathway by adiponectin and insulin in mouse adipocytes: requirement of acyl-CoA synthetases FATP1 and Acsl1 and association with an elevation in AMP/ATP ratio. Faseb. J. 24, 4229–4239 (2010).
Rotter, V., Nagaev, I. & Smith, U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 278, 45777–45784 (2003).
Olefsky, J. M. & Glass, C. K. Macrophages, inflammation and insulin Resistance. Annu. Rev. Physiol. 72, 219–246 (2010).
Folch, J., Lees, M. & Sloane Stanley, G. H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Acknowledgements
This research was supported by Technology Commercialization Support Program (Program no. 1130373), Ministry of Agriculture, Food and Rural Affairs and SRC program (Center for Food & Nutritional Genomics: Grant no. 2015R1A5A6001906) of the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology.
Author information
Authors and Affiliations
Contributions
T.T., Y.S. and T.P. designed the experiments, researched and analyzed data and wrote the manuscript. H.W.L. and R.Y. contributed to experimental design and reviewed and edited the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tong, T., Shen, Y., Lee, HW. et al. Adenylyl cyclase 3 haploinsufficiency confers susceptibility to diet-induced obesity and insulin resistance in mice. Sci Rep 6, 34179 (2016). https://doi.org/10.1038/srep34179
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34179
This article is cited by
-
α-Cedrene protects rodents from high-fat diet-induced adiposity via adenylyl cyclase 3
International Journal of Obesity (2019)
-
ADCY3, neuronal primary cilia and obesity
Nature Genetics (2018)
-
Loss-of-function mutations in ADCY3 cause monogenic severe obesity
Nature Genetics (2018)
-
Olfactory receptor 10J5 responding to α-cedrene regulates hepatic steatosis via the cAMP–PKA pathway
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.