Abstract
Francisella tularensis are highly infectious microbes that cause the disease tularemia. Although much of the bacterial burden is carried in non-phagocytic cells, the strategies these pathogens use to invade these cells remains elusive. To examine these mechanisms we developed two in vitro Francisella-based infection models that recapitulate the non-phagocytic cell infections seen in livers of infected mice. Using these models we found that Francisella novicida exploit clathrin and cholesterol dependent mechanisms to gain entry into hepatocytes. We also found that the clathrin accessory proteins AP-2 and Eps15 co-localized with invading Francisella novicida as well as the Francisella Live Vaccine Strain (LVS) during hepatocyte infections. Interestingly, caveolin, a protein involved in the invasion of Francisella in phagocytic cells, was not required for non-phagocytic cell infections. These results demonstrate a novel endocytic mechanism adopted by Francisella and highlight the divergence in strategies these pathogens utilize between non-phagocytic and phagocytic cell invasion.
Similar content being viewed by others
Introduction
The potentially fatal disease tularemia is caused by the pathogenic microbe Francisella tularensis subspecies tularensis (F. tularensis). These bacteria are highly virulent as exposure to as few as 10 organisms can result in 3035% mortality if left untreated1. F. tularensis enter hosts through a variety of routes including inhalation, ingestion, abrasion and transmission through arthropod vectors. Within their hosts these microbes colonize a variety of organs including the lungs, spleen and liver2,3. F. tularensis encode a secretion system that shares homology with type VI secretion gene clusters, as well as crucial genes needed for intracellular growth and virulence on a region of their bacterial chromosome called the Francisella Pathogenicity Island4,5. Much of the work examining the genetics, biochemistry and cell biology of F. tularensis exploit two closely related surrogate F. tularensis subspecies: Francisella tularensis subspecies novicida (F. novicida); a murine pathogen that does not normally cause disease in healthy humans6 but colonizes identical organs as F. tularensis during in vivo murine infections7 and infects both phagocytic4 and non-phagocytic cells6,8,9,10. F. novicida and F. tularensis share >97% of their DNA sequence6 and both have homologous virulence factors. The secoond surrogate commonly used is F. tularensis subspecies holarctica Live Vaccine Strain (Francisella LVS); an attenuated strain that can infect human and murine cells8,9,11.
Microbes often utilize multiple strategies to gain entry into cells. The process of endocytosis encompasses both the internalization of extracellular particles into phagocytic cells by phagocytosis and non-phagocytic cells referred to as pinocytosis12. These entry methods center around the use of the large GTPase, dynamin II, in releasing the endocytic vesicles from the invaginating membrane at the final stage of endocytosis (scission). Dynamin dependent pinocytosis is sub-divided into two categories; clathrin-mediated or caveolin-mediated pinocytosis12. Clathrin-mediated pinocytosis uses the coat protein clathrin and a number of accessory proteins to create a structure known as a clathrin-coated vesicle (CCV) for internalization. Although the mechanism of pinocytosis was thought to be restricted to the internalization of vesicles ranging from 30nm to150nm in diameter, recent work has demonstrated that bacteria and ligand coated beads up to 5.5m are readily internalized into cells via clathrin-mediated endocytosis13. Caveolin-mediated endocytosis utilizes caveolin-1, a cholesterol-binding integral membrane protein, to form 5080nm flask-shaped membrane invaginations called caveolae. Caveolae are also associated with sphingolipid and cholesterol-rich sub-domains, commonly called lipid rafts14. Consequently, perturbation of cholesterol or lipid rafts often impede caveolin-dependent endocytosis15. Dynamin-independent pinocytosis has emerged as an internalization strategy utilized by many microbes16,17,18. This type of pinocytosis can be separated into three general divisions: 1) non-clathrin/non-caveolin dependent pinocytosis, 2) macropinocytosis and 3) lipid raft-mediated pinocytosis14.
Like many invasive pathogens, F. tularensis gain entry into host cells as an initial step of infection. Efficient internalization of F. tularensis in macrophages has been shown to require complement19. Additionally, through the use of the cholesterol sequestering agent Methyl--cyclodextran in combination with a plasma membrane marker, it has been suggested that cholesterol-rich microdomains of the plasma membrane, also known as lipid rafts20 are also involved in F. tularensis internalization into phagocytes20,21. These factors together with the endocytic protein caveolin-1 are thought to synergistically participate in the invasion of Francisella in phagocytic cells20. Although Francisella has been known to invade non-phagocytic cells8,9,10, the mechanism of Francisella entry into these types of cells has not yet been elucidated. In order to examine the sub-cellular mechanisms Francisella use to invade hepatocytes, we developed two F. novicida infection models using two different non-phagocytic murine hepatocyte cell lines, NMuLi and BNL CL.2 cells. Through the coupling of our in vitro systems with pharmacological inhibitors and RNA interference (RNAi), we demonstrate that the clathrin-associated machinery together with cholesterol are vital for efficient F. novicida invasion into non-phagocytic cells and that this internalization is independent of caveolin.
Results
Murine hepatocytes, a target of F. novicida
F. tularensis are known to infect both phagocytic22,23,24,25 and non-phagocytic host cells9,11,26; however studies examining non-phagocytic epithelial cells during these infections have trailed those of phagocytes. In order to generate a baseline for comparison we initially examined F. novicida infections occurring within murine hepatocytes by using the common F. novicida infection strategy of intraperitoneally infecting mice with 50 F. novicida27,28. At 48h post-infection, livers were collected from mice and subsequently co-stained with anti-albumin antibodies, a classical marker of hepatocytes29,30,31 and anti-F. novicida antibodies. Cells that stained positive for albumin were typically infected in clusters and varied from a few bacteria within a single cell to an unmesurable number of F. novicida that completely filled the host cytoplasm (Figure 1A). By using microscopy and manually enumerating cells infected with F. novicida in liver cross sections, we found that 11.3% of albumin-positive cells and 1.1% of albumin-negative cells (those that did not react with anti-albumin antibodies) were infected (Figure 1B). This work thus sets a benchmark for the establishment of our epithelial cell culture models using F. novicida and suggested that the majority of infected cells within the livers of F. novicida infected mice were hepatocytes.
F. novicida colonization of murine hepatocytes.
(A) Immunofluorescent and phase micrographs showing wild-type F. novicida, albumin and DNA DAPI localization in infected liver tissue section harvested from mice challenged via the intraperitoneal route. Arrows indicate uninfected albumin-negative cells, large arrowheads point to infected albumin-positive cells containing uncountable levels of F. novicida and the small arrowhead points to an albumin-positive cells cells with few internalized bacteria respectively. Scale bar = 10m. (B) Quantification of cells infected with F. novicida based on immunofluorescent images of tissue sections stained with anti-mouse albumin and anti F. novicida antibodies. n (below) is defined as the number of tissue section regions collected in which image stacks were taken (each image stack represents between 500 to 1500 cells). Mouse 1 (n = 17), Mouse 2 (n = 21) and Mouse 3 (n = 17). *** P<0.0001.
To generate cell culture models that would be useful for studying F. novicida hepatocyte infections, we selected two non-phagocytic hapatocyte cell lines, BNL CL.2 and NMuLi cells. We confirmed their hepatocyte characteristic by immunolocalizing albumin in those cell lines and found that both cell lines contained the classical albumin staining present in hepatocytes (Figure S1). Following that verfication, both cell types were infected at a multiplicity of infection (MOI) of 100 for 24h. During these infections the number of intracellular bacteria varied from <10, to hundreds of bacteria within the cytosol of a single cell (Figure 2A). Approximately 15.8% of BNL CL.2 and 25.4% of NMuLi cells became infected with F. novicida (Figure 2B) and the bacterial clustering patterns observed in vivo were also evident in the in vitro results (Figure 1A, 2A). Next, we examined the colonization levels of F. novicida during shorter infection durations and coupled microscopic examinations with invasion assays using both cell lines. We found a modest level of invasion at 4h and 8h (104CFU/mL), whereas the highest level of intracellular bacteria were detected at 24h of infection (106CFU/mL) (Figure 2A), suggesting that the bacteria had invaded the hepatocytes and likely replicated within them.
F. novicida invades and replicates within BNL CL.2 and NMuLi hepatocytes.
(A) Immunofluorescent images of F. novicida infections in cultured hepatocytes. Hepatocytes were fixed following 24 h F. novicida infections with both BNL CL.2 and NMuLi cells. Samples were labelled with anti-F. novicida antibodies (green), fluorescent phalloidin to indicate the cell boundaries (red) and DAPI (blue). Arrowheads indicate some of the bacteria within the infected cells. Scale bar = 10m. (B) Quantification of the proportion of infected hepatocytes as assessed by microscopic examination. NMuLi (n = 17) and BNL CL.2 (n = 16). (C) Titre of intracellular F. novicida at various timepoints following infection of BNL CL.2 and NMuLi cell infections by invasion assay (n = 3).
Francisella enters non-phagocytes through a clathrin and cholesterol-dependent mechanism
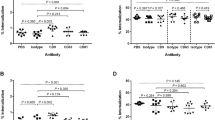
Diverse endocytic strategies are used by microbes to gain entry into non-phagocytic cells32. To evaluate the internalization mechanism(s) that promote F. novicida invasion of hepatocytes, we used invasion assays together with pharmacological inhibitors that disrupt specific cellular components to block particular endocytic functions. Clathrin-mediated endocytosis (CME) is a classical pathway often used by pathogens to gain entry into non-phagocytic cells (extensively reviewed by others33 and our lab32). To determine whether CME was involved in F. novicida invasion, we analyzed levels of bacterial internalization in the presence of the chemical inhibitors monodansylcadaverine (MDC) and chlorpromazine (CPZ), which are frequently used to study the role of CME during pathogen invasion34,35. MDC is a transglutaminase inhibitor that prevents the assembly of clathrin-coated pits (CCP) at the plasma membrane, whereas CPZ functions as a clathrin-sequestering agent, inhibiting endosomal recycling of clathrin. We pre-treated both NMuLi and BNL CL.2 cells with CPZ and MDC at various concentrations for 30 minutes prior to infections with F. novicida and analyzed their effects on bacterial invasion. F. novicida internalization into host cells was reduced at 80M MDC (by 60% ) and 5M CPZ (by 4050%) as compared to untreated cells (Figures 3A, 4A). Neither condition caused any detectable cytotoxicity to host cells or bacteriocidal effects as drug treated cells remained morphologically viable and intact when observed by phase contrast microscopy (data not shown). Because these drugs are well-known inhibitors that are specific for blocking CME, the loss of bacterial invasion in drug treated cells suggested that F. novicida invasion likely occured through a CME pathway.
Internalization of F. novicida into BNL CL.2 cells is a clathrin- and cholesterol-dependent process that requires actin.
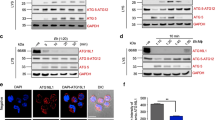
(AD) BNL. CL.2 cells were pre-treated with the indicated drugs and incubated with F. novicida for 22 h followed by a 2 h gentamicin treatment for CFU enumeration. (A) Clathrin inhibitors: 80M monodansylcadaverine (MDC) or 5.0M chlorpromazine (CPZ). (B) Cholesterol inhibitors: 2.5mM MCD or 80M MDC supplemented with 1mM cholesterol. (C) Individual or a combination of 2.5mM MCD and 80M MDC. (D) Cytoskeleton inhibitors: 5M cytochalasin D (CytD), 2.5M latrunculin A (LN A) or 2.5M nocodazole (ND), * P<0.01. ** P< 0.05. (E) Western blot shows that the overall level of clathrin heavy-chain (HC) protein remains unaltered after 24 h F. novicida infections of BNL CL.2 cells compared to uninfected cells. For immunolocalization experiments, untreated cells were infected with F. novicida for 8h, fixed and stained with (F) an anti-F. novicida antibody (green), anti-clathrin HC (red) and DAPI or (G) an anti-F. novicida antibody (green) and filipin (blue) as well as phase contrast. Arrowheads indicate some of the bacteria thatco-localize with the other labels. Scale bar = 5m.
Internalization of F. novicida into NMuLi cells is a clathrin- and cholesterol dependent process that requires actin.
NMuLi cells were pre-treated with the indicated drugs and incubated with F. novicida for 22 h followed by a 2 h gentamicin treatment for CFU enumeration. (A) Clathrin inhibitors: 80M monodansylcadaverine (MDC) or 5.0M chlorpromazine (CPZ). (B) Cholesterol inhibitors: 2.5mM MCD or 80M MDC supplemented with 1mM cholesterol. (C) Individual or combination of 2.5mM MCD and 80M MDC. (D) Cells were treated simultaneously with 2g/mL progesterone and 10M nystatin (Prog/Nys). Cytoskeleton inhibitors: 5M cytochalasin D (CytD), 2.5M latrunculin A (LN A) * P<0.01, ** P<0.05.
Despite the presence of the CME blocking agents, F. novicida still exhibited the ability to enter epithelial cells (Figure 3A, 4A), suggesting that F. novicida likely use alternative route(s) in addition to CME for entry. To investigate how F. novicida could invade epithelial cells with dysfunctional clathrin machinery, we examined clathrin-independent endocytic mechanisms, such as caveolin and lipid-raft dependent endocytosis, both of which require cholesterol-rich domains36,37. Extraction of cholesterol from the plasma membrane using methyl--cyclodextrin (MCD), a potent inhibitor that disrupts lipid composition and thus cholesterol-based endocytosis20,38, caused a statistically significant reduction, of 60%, in F. novicida invasion (Figure 3B, 4B). We performed additional tests using a combination of progesterone (a cholesterol-synthesis inhibitor) and nystatin (a cholesterol-sequestering agent) Prog/Nys to validate the importance of lipid raft domains during these invasion events. Interruption of synthesis and transport of cholesterol to membrane lipid-raft domains by these drugs caused a moderate (30%), yet statistically significant inhibition in F. novicida uptake in epithelial cells (Figure 4D). This data suggests that the presence of cholesterol at the plasma membrane is necessary for efficient invasion given that removal of cholesterol caused more prominent defects in invasion as compared to lipid-raft disruption through cholesterol binding. The importance of cholesterol at the plasma membrane for F. novicida invasion was further emphasized when the invasion levels were almost restored to those of untreated cells when cells that were pre-treated with MCD were supplemented with excess cholesterol prior to the infections (Figure 3B). By contrast, replacement of cholesterol failed to enhance F. novicida invasion in MDC treated cells. To further analyze the importance of cholesterol and CME during F. novicida invasion, a combination of MCD and MDC were used concurrently to deplete both cholesterol and clathrin in host cells, thus blocking both CME and cholesterol-dependent endocytosis. This further exaggerated the inhibitory effect of F. novicida internalization when compared to a single inhibitor treatment, as the invasion level was diminished by 80% (Figure 3C). We also explored other possible endocytic pathways such as caveolin-dependent endocytosis and/or macropinocyosis that might be used by F. novicida. To accomplish this, we infected cells in the presence of filipin, a cholesterol-sequestering agent that impairs the membrane invagination of caveolae and consequently prevents caveolin-based endocytosis37. F. novicida invasion appeared to be resistant to filipin treatment as it did not significantly impede invasion levels in either BNL CL.2 or NMuLi hepatocytes (Figure 5A). Further analysis by immunolocalization also revealed a normal pattern of caveolin at the cell periphery and within the cytoplasm that resembled untreated cells; the cytoplsmic caveolin did not accumulate near F. novicida throughout different stages of infection at 8h, 16h or 24h post-infection (Figure 5B). These results strongly indicate that caveolin likely does not participate in F. novicida invasion of hepatocytes. To assess whether F. novicida engages in macropinocytosis during invasion, we examined cellular invasion in the presence of amiloride, an inhibitor of the Na+/H+ exchange channel at the plasma membrane known to specifically block internalization via macropinocytosis39. Treatment with amiloride did not cause any notable changes in F. novicida invasion, especially when compared to Salmonella Typhimurium invasion, which uses well established macropinocytosis strategies to invade their host's cells (Figure 5C)40,41. This result is consistent with the lack of actin membrane ruffles (a characteristic of macropinocytosis) in the vicinity of F. novicda during the invasion process (Figure 2A), thus strengthening the evidence that F. novicida does not utilize macropinocytosis during its invasion process of hepatocytes.
F. novicida entry does not utilize caveolin-dependent or macropinocytosis pathways.
(A) BNL CL.2 and NMuLi cells were treated with the caveolin-inhibitor filipin and infected with F. novicida for 22 h followed by 2 h gentamicin treatment for invasion assays. (B) Immunolocalization of caveolin-1 (green) and DAPI (blue) of uninfected BNL CL.2 cells or following 8 h, 16 h and 24 h infections with F. novicida showed no co-localization between F. novicida and caveolin-1. Arrowheads indicate the localization of F. novicida. Scale bar = 5m. (C) 5mM of amiloride was used to pre-treat BNL CL.2 cells and HeLa cells for 15 min prior to infection with F. novicida and Salmonella Thyphimurium (SL1344), respectively. Untreated cells were treated with media containing DMSO. Cells infected with F. novicida for 6 h followed by 2 h gentamicin treatment did not show a decrease in invasion while cells infected with S. Typhimurium for 30 min followed by 1 h gentamicin treatment showed a significant decrease in invasion *P<0.01.
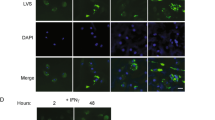
Having demonstrated that clathrin and cholesterol inhibitors significantly attenuate F. novicida invasion, we extended our study by immunolocalizing clathrin and cholesterol to assess whether they became re-distributed during this process. To visualize clathrin localization during invasion, BNL CL.2 cells were infected with F. novicida for 8h and co-localized with anti-F. novicida and anti-clathrin antibodies. We chose the 8h timepoint in an attempt to identify regions of bacterial invasion without significant cytoplasmic bacterial replication so that the precise localization of the proteins could be identified at sites of bacterial contact. We found that while the overall protein levels of clathrin remained unchanged (Figure 3F), clathrin localized strongly at sites where F. novicida interacted with the host cells (Figure 3E). This finding was also evident in BNL CL.2 cells during Francisella LVS invasion as clathrin was found to associate closely with invading Francisella LVS following 8h infections (Figure S2). To examine the distribution of cholesterol, we took advantage of the cholesterol binding property of the fluorescent probe filipin36,42. Similar to clathrin staining, we observed an accumulation of filipin at sites of F. novicida invasion (Figure 3F). Altogether, our data suggest that F. novicida invades epithelial cells at cholesterol-rich domains, in a clathrin-dependent manner.
Clathrin accessory proteins Eps15 and AP-2 are recruited and required for efficient Francisella invasion
To investigate whether F. novicida utilize the protein assembly of classical-CME machinery for cell invasion, we examined the roles of CME adaptor proteins during F. novicida invasion of non-phagocytic cells. Using a similar approach as aforementioned, we immunolocalized Eps15 and AP-2 (housekeeping components that typically participate in cargo recognition and CCP formation to facilitate the progression of CME43) 8hours post-infection and found that both Eps15 and AP-2 were recruited to sites of invasion and co-localized with F. novicida in a similar pattern as was found with clathrin (Figure 6A, 6D). Identical results were also found during Francisella LVS infections as bacteria were co-localized with cellular Eps15 and AP-2 after 8h infections (Figure S2). Eps15 or AP-2 protein expression levels remained unchanged in cells infected with F. novicida when compared to uninfected cells (Figure 6B, 6E). Because both AP-2 and Eps15 are major constituents of CCPs and govern the progression of CME, recruitment of these molecules to sites of Francisella interaction with host cells strongly supports the notion that Francisella exploit CME as a route for epithelial cell entry.
Clathrin-associated adaptor proteins Esp15 and AP-2 are crucial for F. novicida invasion into non-phagocytic cells.
(A, D) BNL CL.2 cells were infected with F. novicida for 8 h and immunolocalized with anti-F. novicida together with (A) anti-Eps15 (red) or (D) anti-AP-2 -adaptin (green) antibodies. Arrowheads indicate bacteria and protein co-localization. Scale bar = 5m. (B and E). Western blots show the overall protein levels of (B) Eps15 (145kDa) or (E) AP-2 -adaptin (50kDa) in 8 h F. novicida infected cells (untreated with RNAi) remained unchanged compared to uninfected control. Cells transiently transfected with siRNA (48 h) showed a significant reduction in the targeted Eps15 or AP-2 -adaptin proteins as compared to cells transfected with siRNA control pool (CP). (C and F) Reduced Eps15 and AP-2 -adaptin lead to a significant defect in F. novicida internalization 24 h post-infection. *P<0.05.
We continued our study by investigating the importance of Eps15 and AP-2 in F. novicida invasion. Using RNAi, we knocked down both Eps15 and AP-2 (-adaptin subunit) to undetectable levels (Figure 6B, 6E). Depletion of these proteins have been shown to impede CME of many extracellular cargos, including microbes44. We found that loss of Eps15 caused a significant inhibition in clathrin-dependent internalization of F. novicida, resulting in only 20% invasion relative to cells treated with non-targeting control pool (CP) siRNA (Figure 6C). To confirm the importance of clathrin-coat formation at the plasma membrane during the invasion process, we knocked down AP-2, which is known to cause an interruption in CCP assembly. Consistent with results from Eps15 RNAi, AP-2 depleted cells also exhibited a significant decrease in F. novicida invasion, with 40% of invasion remaining when compared to untreated cells (Figure 6F). Collectively, these data show that the clathrin adaptors AP-2 and Eps15 are essential for clathrin-dependent uptake of F. novicida.
Disruption of actin polymerization interferes F. novicida internalization
The cytoskeleton is known to be an integral element necessary for functional and efficient endocytosis45,46. Using the cytoskeletal targeting drugs, cytochalasin-D (Cyt D) and colchicines, Craven and co-workers had previously demonstrated that both actin filaments and microtubules were used by Francisella LVS to efficiently invade pulmonary epithelial cells8. To assess whether F. novicida required these filament systems for efficient invasion in hepatocytes, we utilized the cytoskeletal inhibitors Cyt D and latrunculin A (LN A) to block actin filament polymerization. Additionally, we used nocodazole, a pharmacological drug that interferes with microtubule polymerization. Consistent with a previous Francisella LVS report in alveolar epithelial cells47, we found that cells pre-treated with the actin-disrupting agents exhibited a significant defect in F. novicida invasion in hepatocytes. Cells pre-treated with Cyt D or LN A blocked >60% of bacterial entry as compared to untreated cells (Figure 3D, 4D). In contrast, nocodazole treated cells did not exhibit any significant defects in internalizing F. novicida (Figure 3D). Together, these results suggest that the actin machinery, but not microtubules, is needed for efficient F. novicida invasion into hepatocytes. To further delineate the role of actin during F. novicida invasion, we immunolocalized actin filaments at 8h and 24h of infection and detected no direct association of actin and F. novicida at either time point (Figure 2). Consequently, it appears that F. novicida either uses actin for invasion via an indirect manner or could use it as part of the CME process, as the actin cytoskeleton has been described to play an important role in facilitating CME46.
Discussion
Over the past number of years a familiar theme of endocytic protein hijacking by microbial pathogens has developed. Although Francisella has been identified as an invasive intracellular pathogen2,3, a general lack of knowledge of its internalization process and lifestyle in non-phagocytic cells remains. Most of the previous Fransicella invasion studies into non-phagocytic cells have been focused on examinations of the Francisella live vaccine strain (LVS). One study demonstrated that the Francisella LVS can invade and replicate within murine alveolar cells in vivo, as well as in TC-1 and MLE 12 murine lung epithelial cell lines and human alveolar type2 cells A5498. Another study demonstrated that the Francisella LVS can infect Hep-2 and human bronchial epithelial HBE cells in vitro; invasion assays showed relatively low levels of Francisella LVS invasion in these cells 5hours post-infection, as approximately 5103104CFU were recovered, representing 0.05%0.1% of the initial inoculum47. Although these important studies demonstrated the ability of Francisella LVS to replicate within these cell types, the internalization strategies utilized by these microbes during their invasion events still required further investigation. Because of the fastidious nature of the Francisella LVS, we opted to primarily use F. novicida as a model of F. tularensis infections. Using these bacteria we demonstrated a high level of colonization in murine heptaocyte in vivo and to elucidate their mechanisms of entry we developed 2 robust in vitro hepatocyte infection models.
We initiated our study by utilizing a panel of drugs that target specific endocytic pathways; none of the drugs used in the study caused adverse effects in bacterial growth, which suggested that the inhibitory effects we observed specifically impeded bacterial entry instead of bacterial replication. Although centrifugation of bacteria onto the host cells is a strategy that some laboratories use to enhance host cell contact with the microbes, in our experiments we allowed the bacteria to attach and colonize onto the surface of host cells without external forces to decrease the external influences during the infections. After monitoring levels of F. novicida infection in the heptaocytes throughout a 24h period, we found a moderate degree of bacterial containment within the hepatocytes at the 8h time point and not surprisingly a much higher level of invasion 24h post-infection. Unlike many fast-invading bacteria such as Salmonella Typhimurium, which invade within 30 minutes40, F. novicida required an extended amount of time to initiate invasion into host cells for suitable detection and analysis. As a result, we chose to primarily monitor F. novicida invasion for up to 24h infections to have ample numbers of internalized bacteria for comparison. Extended infection durations are not uncommon, as Helicobacter pylori invasion into cultured cells occurs at 24h post-infection48 and Chladmydia trachomatis invasion is often assessed between 24h to 72h post-infection49,50. Using MDC and CPZ, we first found that the loss of functional CME caused a significant decrease in F. novocida invasion when asessed at 24h. Furthermore, we identified clathrin and major associated proteins at the Francisella entry sites after 8h of infection. Taken together, these results suggest that CME is a key pathway targeted by these microbes, regardless of the endopoint assayed.
CME is a complex and multi-step process that involves many clathrin accessory proteins to complete the process. Among the 20 accessory proteins, Eps15 and AP-2 have been shown to be important for efficient internalization of molecules in general51. Our finding of impaired F. novida invasion following Eps15 and AP-2 siRNA treatment highlights the importance of these clathrin-mediated endocytic proteins and CME as a whole during Francisella invasion.
Because F. novicida retained some capacity to invade these epithelial cell lines in the absence of functional CME, we postulated that another strategy must also be involved. Using the strong cholesterol-binding agent MCD we extracted cholesterol from the membrane and found a significant decrease in F. novicida uptake. This phenotype reverted when the samples were supplemented with excess cholesterol. These findings coupled with the observation of cholesterol at F. novicida entry sites is reminiscent of cholesterol recruitment seen during Shigella flexneri invasion of HeLa cells42.
Because phagocytic cells have been shown to use caveolin during F. novicida invasion events, we also studied its involvement and found that caveolin was not required for hepatocyte invasion, despite our findings that invasion requires cholesterol. Such dependence on cholesterol-rich domains in the absence of caveolin is not exclusive to F. novicida as it has been previously demonstrated during the invasion of viral (i.e., Simian virus 40)52 bacterial (i.e., Brucella abortus)53, as well as a much larger parasitic (Toxoplasma gondii)54 pathogens.
Recruitment of actin filaments at the site of internalization has been recognized as important processes during endocytosis, as polymerization of actin creates mechanical force to promote membrane curvature and invagination55. In this study, we have shown that F. novicida invasion is severely inhibited in the presence of the actin inhibitor cytochalasin D without detecting any notable membrane ruffling as seen during Salmonella invasion; similar results were previously described in a Francisella LVS invasion study using HEp-2 cells47 and murine alveolar cells TC-18. While it is likely that F. novicida invasion does not induce massive actin reorganization at the invasion sites, it has also been challenging to detect the small amounts of actin recruited to sites of endocytosis using fluorescent microscopy. Based on the involvement of actin in endocytosis46,55, we suspect that the inhibition of invasion through the impairment of the actin cytoskeleton likely comes from the association of the actin meshwork and endocytic events46. While the same Francisella LVS studies also suggested Francisella entry into TC-1 lung epithelial cells and human HEp-2 epithelial cells required microtubule8,47, we did not detect any notable defects in F. novicida invasion into either NMuLi or BNL.Cl2 cells when infected with F. novicida in the presence of the microtubule disrupting drug nocodazol. This discrpency could be due to the difference in the Francisella strain and host cell types used.
Collectively, we have demonstrated F. novicida uses multiple strategies for efficient internalization into hepatocytes, which involves functional clathrin-mediated endocytosis and cholesterol-rich microdomains.
Methods
Bacteria and growth conditions
F. tularensis subsp. novicida strain U112 was grown on trypticase soy agar (TSA) or broth (TSB) supplemented with 0.1% L-cysteine at 37C. The bacteria were grown to stationary phase for 18h at 37C with shaking at 220rpm. Salmonella Typhimurium (strain SL1344) was grown using the same parameters on Luria Bertani (LB) agar or broth.
Animal infections, tissue preparation and tissue immunolocalization
Female BALB/C mice aged 68 weeks (Charles River Labortories, Quebec, Canada) were left undisturbed to recover from transport for at least 4 days. Infections were performed by intraperitoneal injection of 5010 F. novicida bacteria in 100l of TSB + 0.1% L-cysteine. Immediately following the infection, the identical volumes of the bacterial culture were spread onto TSA-C plates. Colony forming units (CFUs) were enumerated after plates were incubated overnight at 37C. Following 48h infections, mice were euthanized and livers extracted then submerged into 3% paraformaldehyde (room temperature) in phosphate-buffered saline (PBS; 150mM NaCl, 5mM KCl, 0.8mM KH2PO4, 3.2mM Na2HPO4, pH7.3) for 3h. After 3 successive 10min PBS washes, fixed organs were frozen (using liquid nitrogen) and attached to an aluminum stub by OCT compound (Sakura Finetek USA, Torrance). 5m frozen sections were cut and attached to Superfrost (VWR) glass slides that were pre-coated with 0.01% poly-L-lysine solution (Sigma). Slides with attached tissue sections were immediately plunged into 20C acetone for 5min, air-dried, then blocked with 5% Blotto non-fat dry milk (Santa Cruz Biotechnology) in PBS for 20min. The slides were then incubated overnight at 4C with a 11000 dilution of rabbit anti-F. novicida antibodies4,56 and 20ng/l goat anti-mouse albumin (Bethyl Laboratories Inc.) antibodies diluted in PBS containing 1% Tween-20 and 1% non-fat milk. The following day the sections were washed 3 times for 10min each with TPBS/non-fat milk (1% Tween-20 and 0.1% non-fat milk in PBS) before being incubated for 2h with 20ng/l Alexa Fluor 594 donkey anti-goat antibodies (Invitrogen) then with 20ng/l Alexa Fluor 488 goat anti-rabbit antibodies for 1h (Invitrogen). After the last set of washes, coverslips were mounted using Prolong Gold (Invitrogen). Images were acquired using a Leica DMI4000B inverted fluorescent microscope equipped with a Hamamatsu Orca R2 CCD camera (Hamamatsu, Japan) and Metamorph Imaging System software (Universal Imaging Corp., Pennsylvanian). The presence of albumin in cells was asessed by examining samples and those that did not react were considered absent of albumin. In all of those instances positively reacting cells were also present in the samples, which acted as positive controls for the experiment. For antibody specificity controls, primary antibodies were replaced with normal immunoglobulin from the host animal species at identical concentrations to the primary antibodies.
Cell lines and culture model infection parameters
Cultured BNL CL.2 (TIB-73) and NMuLi (CRL-1638) murine hepatocytes (ATCC) were grown in high-glucose Dulbecco's modified Eagle's medium (DMEM) (Thermo Scientific) supplemented with 10% Fetal Bovine Serum (FBS) at 37C and 5% CO2. Cells were grown to confluence in 6- or 24-well cell culture plates overnight in 10% FBS in DMEM before switching to 10% FBS in DMEM supplemented with 0.1% L-cysteine prior to infection.
Cultured cell invasion assays and inhibitor studies
Inhibitors targeting different endocytic pathways were used to evaluate levels of F. novicida invasion. The duration of treaments and concentrations were determined and optimized according to previous studies as well as cell viability assays. BNL CL.2 or NMuLi cells were seeded at a concentration of 1.5105cells/mL in 24-well culture plates and used 24h after seeding. Monolayers of confluent cells were pre-incubated at 37C with the following inhibitors for 30min: 5M chloropromazine, 5M cytochalasin D, 2.5M lantruculin A, 2.5mM MCD, 80M MDC, 2g/mL progesterone combined with 10M nystatin. Cells were pre-treated for 2h with 2.5M nocodazole and 15min with 5mM amiloride at 37C prior to infection. For the cholesterol supplementation study, 1mM solubilized cholesterol in chloroform was added to MCD treated cells at a final concentration of 2mM for 30min prior to infection. All compounds (purchased from Sigma) were maintained in the media during infections except amiloride, which was removed prior to infections. Untreated controls are only treated with carrier buffer (ie: DMSO) in the absence of inhibitors. Following pre-treatment, cells were infected with F. novicida at an MOI of 100. At 22h post-infection, cells were washed three times with warm DMEM + 10% FBS and treated with media containing 100g/ml gentamicin for 2h at 37C to kill extracellular bacteria. For amiloride studies, cells were infected with F. novicida at an MOI of 100 for 6h followed by 2h gengamicin treatment. As a positive control, HeLa cells were infected with Salmonella Typhimurium at an MOI of 100 for 30min followed by 1h gentamciing treatement. After 3 PBS washes, cells were lysed either mechanically using a 271/2 gauge needle or chemically using 1% Triton X-100 in PBS. The released internalized bacteria were immediately serially diluted and plated on TSA-C for colony-forming unit (CFU) enumeration to quantify levels of internalization.
Cultured cell immunolocalization
Cells were grown to 80% confluency on sterile No.1.5 glass coverslips in 6-well culture plates. Prior to infection, media was replaced with DMEM + 10% FBS with 0.1% cysteine. After 8h, 16h or 24h infections at an MOI of 100, cells were fixed in 3% paraformaldehyde in PBS for 15min, washed three times with PBS, incubated with 0.2% Triton X-100 and treated with 5% NGS following chemical reagent or antibody treatments in processes mentioned above. Cholesterol localization was determined using a UV fluorescent cholesterol binding fungal toxin, filipin, which binds cholesterol and emits blue fluorescence. Cells were incubated with filipin (50g/mL) for 30min at RT, washed three times with PBS, then co-localized with F. novicida. Clathrin, Eps15, AP-2 and caveolin-1 localization was performed using mouse anti-clathrin heavy chain antibodies at 15.6g/ml (BD Biosciences), mouse anti-Eps15 (clone 17) antibodies at 16.67g/ml (Santa Cruz Biotechnology), mouse anti-AP2 -adaptin (3B5) at 10g/ml (BD Biosciences), or mouse anti-caveolin-1 (clone 2297) antibodies at 16.67g/ml (BD Biosciences). Prior to staining with clathrin, Eps15 and AP-2 antibodies, the antibodies were pre-cleared against F. novicida to ensure no cross reactivity with the bacteria. To accomplish this, 1ml of live F. novicida was pelleted, fixed for 20min with 500l of 3% paraformaldehyde in PBS at RT then washed 6 times with PBS at 15min intervals. F. novicida was pelleted and re-suspended overnight at 4C with the antibody, prepared at the concentrations described above, then used for immunolocalization.
Cell lysate preparation and western blotting
BNL CL.2 cells were grown on 150mm tissue culture dishes and infected at an MOI 100 with F. novicida for 24h. Cells were washed 3 times with PBS containing 1mM CaCl2 and 1mM MgCl2 followed by treatment with RIPA lysis buffer (150mM NaCl, 50mM Tris pH7.4, 5mM EDTA, 1% Nonidet P-40, 1% Deoxycholic acid, 10% SDS) for 10min on ice. Western blotting was performed according to Guttman and colleagues57. Briefly, equal amounts of total protein was loaded and separated on 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad Laboratories). Membranes were blocked with 5% non-fat milk and washed in Tris-buffered saline with 0.1% Tween-20 (TBST) 3 times for 5min. A primary rabbit anti-Eps15 antibody (Santa Cruz Biotechnology, CA) was used at 1.0g/ml, a mouse anti-AP2 -adaptin (3B5) antibody (Trevor Williams lab, Developmental Studies Hybridoma Bank, IA) was used at 0.725g/ml and a mouse anti-clathrin heavy chain antibody (BD Biosciences) was used at 0.25g/ml. Antibodies were prepared in TBST supplemented with 1% BSA. After washing, a horseradish peroxidase (HRP)-conjugated secondary antibody (Cell Signaling Technology) was used as previously described57. Signals were detected by enhanced chemiluminescence (Perkin Elmer) and exposed on Kodak BioMax light film.
RNA interference
Small interfering RNA (siRNA) ON-TARGETplus SMART pools were synthesized and purchased from Dharmacon, targeting Eps15 (Catalog: 004005) and AP-2 A1 -adaptin (Catalog: 11771). For RNAi controls, equal amount of siGENOME Non-Targeting siRNA Pool (Catalog: D-001206-13) was used. BNL CL.2 cells were grown in 24 well plates to 40% confluency and transfected with 50nM of the siRNA pool using the jetPRIME transfection reagent or INTERFERin (Polyplus transfection) according to the manufacturer's procedures. At 48h post-transfection, cells were either treated with RIPA lysis buffer supplemented with a protease inhibitor cocktail (Roche) for cell lysates, or infected with F. novicida for 24h before being processed for invasion assays.
Statistical analysis
All experiments were performed at least in triplicate during a single run and repeated a minimum of three times. For enumerating infected murine liver cells, phase and fluorescent images of liver cells stained for albumin (or F-actin), F. novicida and DNA were overlayed and tallied using Fiji software (http://fiji.sc/wiki/index.php/Fiji). Localization of F. novicida within the cytoplasm of albumin-positive and negative cells were considered infected, whereas cells that show no F. novicida localization within the cell periphery were counted as uninfected. Each field of view was acquired as a stack under both phase-contrast and fluorescent microscopy. Student's t-test was applied to determine statistical signifinance between the two experimental groups. For inhibitor studies, results were expressed as the means standard deviation (s.d.) from repeated independent experiments. Differences between untreated and inhibitor treated samples were compared using One-way ANOVA followed by Dunnett's Multiple Comparison Test (P< 0.05). Differences between control pool and RNAi treatments were compared using Student's t test. (P<0.05).
References
Ellis, J., Oyston, P. C., Green, M. & Titball, R. W. Tularemia. Clin Microbiol Rev 15, 631 646 (2002).
Conlan, J. W., Chen, W., Shen, H., Webb, A. & KuoLee, R. Experimental tularemia in mice challenged by aerosol or intradermally with virulent strains of Francisella tularensis: bacteriologic and histopathologic studies. Microb Pathog 34, 239 248 (2003).
Conlan, J. W. & North, R. J. Roles of Listeria monocytogenes virulence factors in survival: virulence factors distinct from listeriolysin are needed for the organism to survive an early neutrophil-mediated host defense mechanism. Infect Immun 60, 951 957 (1992).
Schmerk, C. L., Duplantis, B. N., Howard, P. L. & Nano, F. E. A Francisella novicida pdpA mutant exhibits limited intracellular replication and remains associated with the lysosomal marker LAMP-1. Microbiology 155, 1498 1504 (2009).
Qin, A. & Mann, B. J. Identification of transposon insertion mutants of Francisella tularensis tularensis strain Schu S4 deficient in intracellular replication in the hepatic cell line HepG2. BMC Microbiol 6, 69 (2006).
Santic, M., Al-Khodor, S. & Abu Kwaik, Y. Cell biology and molecular ecology of Francisella tularensis.Cell Microbiol 12, 129 139.
Ojeda, S. S. et al. Rapid dissemination of Francisella tularensis and the effect of route of infection. BMC Microbiol 8, 215 (2008).
Craven, R. R., Hall, J. D., Fuller, J. R., Taft-Benz, S. & Kawula, T. H. Francisella tularensis invasion of lung epithelial cells. Infect Immun 76, 2833 2842 (2008).
Melillo, A. et al. Identification of a Francisella tularensis LVS outer membrane protein that confers adherence to A549 human lung cells. FEMS Microbiol Lett 263, 102 108 (2006).
Mortensen, B. L. et al. Effects of the putative transcriptional regulator IclR on Francisella tularensis pathogenesis. Infect Immun 78, 5022 5032 (2010).
Hall, J. D. et al. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect Immun 76, 5843 5852 (2008).
Doherty, G. J. & McMahon, H. T. Mechanisms of endocytosis. Annu Rev Biochem 78, 857 902 (2009).
Veiga, E. et al. Invasive and adherent bacterial pathogens co-Opt host clathrin for infection. Cell Host Microbe 2, 340 351 (2007).
Lajoie, P. & Nabi, I. R. Regulation of raft-dependent endocytosis. J Cell Mol Med 11, 644 653 (2007).
Thomas, C. M. & Smart, E. J. Caveolae structure and function. J Cell Mol Med 12, 796 809 (2008).
Saeed, M. F., Kolokoltsov, A. A., Albrecht, T. & Davey, R. A. Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog 6, (2010).
Vidricaire, G. & Tremblay, M. J. A clathrin, caveolae and dynamin-independent endocytic pathway requiring free membrane cholesterol drives HIV-1 internalization and infection in polarized trophoblastic cells. J Mol Biol 368, 1267 1283 (2007).
Furuta, N. et al. Porphyromonas gingivalis outer membrane vesicles enter human epithelial cells via an endocytic pathway and are sorted to lysosomal compartments. Infect Immun 77, 4187 4196 (2009).
Clemens, D. L., Lee, B. Y. & Horwitz, M. A. Francisella tularensis enters macrophages via a novel process involving pseudopod loops. Infect Immun 73, 5892 5902 (2005).
Tamilselvam, B. & Daefler, S. Francisella targets cholesterol-rich host cell membrane domains for entry into macrophages. J Immunol 180, 8262 8271 (2008).
Barel, M. et al. A novel receptor-ligand pathway for entry of Francisella tularensis in monocyte-like THP-1 cells: interaction between surface nucleolin and bacterial elongation factor Tu. BMC Microbiol 8, 145 (2008).
Anthony, L. D., Burke, R. D. & Nano, F. E. Growth of Francisella spp. in rodent macrophages. Infect Immun 59, 3291 3296 (1991).
Santic, M., Molmeret, M., Klose, K. E. & Abu Kwaik, Y. Francisella tularensis travels a novel, twisted road within macrophages. Trends Microbiol 14, 37 44 (2006).
Oyston, P. C., Sjostedt, A. & Titball, R. W. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat Rev Microbiol 2, 967 978 (2004).
Geier, H. & Celli, J. Phagocytic receptors dictate phagosomal escape and intracellular proliferation of Francisella tularensis. Infect Immun 79, 2204 2214 (2011).
Ludu, J. S. et al. The Francisella pathogenicity island protein PdpD is required for full virulence and associates with homologues of the type VI secretion system. J Bacteriol 190, 4584 4595 (2008).
Kieffer, T. L., Cowley, S., Nano, F. E. & Elkins, K. L. Francisella novicida LPS has greater immunobiological activity in mice than F. tularensis LPS and contributes to F. novicida murine pathogenesis. Microbes Infect 5, 397 403 (2003)
Tempel, R., Lai, X. H., Crosa, L., Kozlowicz, B. & Heffron, F. Attenuated Francisella novicida transposon mutants protect mice against wild-type challenge. Infect Immun 74, 5095 5105 (2006).
Shanmukhappa, K., Mourya, R., Sabla, G. E., Degen, J. L. & Bezerra, J. A. Hepatic to pancreatic switch defines a role for hemostatic factors in cellular plasticity in mice. Proc Natl Acad Sci U S A, 102, (2005).
Haouzi, D. et al. Three-dimensional polarization sensitizes hepatocytes to Fas/CD95 apoptotic signalling. J Cell Sci 118, 2763 2773 (2005).
Tanimizu, N., Saito, H., Mostov, K. & Miyajima, A. Long-term culture of hepatic progenitors derived from mouse Dlk+ hepatoblasts. J Cell Sci 117, 6425 6434 (2004).
Lin, A. E. & Guttman, J. A. Hijacking the endocytic machinery by microbial pathogens. Protoplasma, 244, 75 90 (2010).
Pizarro-Cerda, J., Bonazzi, M. & Cossart, P. Clathrin-mediated endocytosis: what works for small, also works for big. Bioessays, 32, 496 504 (2010).
Boisvert, H. & Duncan, M. J. Clathrin-dependent entry of a gingipain adhesin peptide and Porphyromonas gingivalis into host cells. Cell Microbiol 10, 2538 2552 (2008).
Acosta, E. G., Castilla, V. & Damonte, E. B. Alternative infectious entry pathways for dengue virus serotypes into mammalian cells. Cell Microbiol 11, 1533 1549 (2009).
Lafont, F. & van der Goot, F. G. Bacterial invasion via lipid rafts. Cell Microbiol 7, 613 620 (2005).
Fielding, C. J. & Fielding, P. E. Relationship between cholesterol trafficking and signaling in rafts and caveolae. Biochim Biophys Acta 1610, 219 228 (2003).
Shin, J. S., Gao, Z. & Abraham, S. N. Involvement of cellular caveolae in bacterial entry into mast cells. Science, 289, 785 788 (2000).
Koivusalo, M. et al. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J Cell Biol 188, 547 563.
Chen, L. M., Hobbie, S. & Galan, J. E. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science, 274, 2115 2118 (1996).
Francis, C. L., Ryan, T. A., Jones, B. D., Smith, S. J. & Falkow, S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature, 364, 639 642 (1993).
Lafont, F., Tran Van Nhieu, G., Hanada, K., Sansonetti, P. & van der Goot, F. G. Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft-mediated CD44-IpaB interaction. EMBO J 21, 4449 4457 (2002).
Traub, L. M. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol 10, 583 596 (2009).
Cureton, D. K., Massol, R. H., Saffarian, S., Kirchhausen, T. L. & Whelan, S. P. Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog 5 (2009).
Samaj, J. et al. Endocytosis, actin cytoskeleton and signaling. Plant Physiol 135, 1150 1161 (2004).
Collins, A., Warrington, A., Taylor, K. A. & Svitkina, T. Structural organization of the actin cytoskeleton at sites of clathrin-mediated endocytosis. Curr Biol 21, 1167 1175 (2011).
Lindemann, S. R., McLendon, M. K., Apicella, M. A. & Jones, B. D. An in vitro model system used to study adherence and invasion of Francisella tularensis live vaccine strain in nonphagocytic cells. Infect Immun 75, 3178 3182 (2007).
Oliveira, M. J. et al. Helicobacter pylori induces gastric epithelial cell invasion in a c-Met and type IV secretion system-dependent manner. J Biol Chem 281, 34888 34896 (2006).
Hybiske, K. & Stephens, R. S. Mechanisms of Chlamydia trachomatis entry into nonphagocytic cells. Infect Immun 75, 3925 3934 (2007).
Stuart, E. S., Webley, W. C. & Norkin, L. C. Lipid rafts, caveolae, caveolin-1 and entry by Chlamydiae into host cells. Exp Cell Res 287, 67 78 (2003).
Traub, L. M. & Lukacs, G. L. Decoding ubiquitin sorting signals for clathrin-dependent endocytosis by CLASPs. J Cell Sci 120, 543 553 (2007).
Damm, E. M. et al. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J Cell Biol 168, 477 488 (2005).
Watarai, M., Makino, S., Fujii, Y., Okamoto, K. & Shirahata, T. Modulation of Brucella-induced macropinocytosis by lipid rafts mediates intracellular replication. Cell Microbiol 4, 341 355 (2002).
Coppens, I. & Joiner, K. A. Host but not parasite cholesterol controls Toxoplasma cell entry by modulating organelle discharge. Mol Biol Cell 14, 3804 3820 (2003).
Kaksonen, M., Toret, C. P. & Drubin, D. G. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 7, 404 414 (2006).
Nano, F. E. Identification of a heat-modifiable protein of Francisella tularensis and molecular cloning of the encoding gene. Microb Pathog 5, 109 119 (1988).
Guttman, J. A. et al. Aquaporins contribute to diarrhoea caused by attaching and effacing bacterial pathogens. Cell Microbiol 9, 131 141 (2007).
Acknowledgements
We would like to thank Karen Lo for her technical assistance. JAG is a CIHR New Investigator. AEL is funded through a CIHR CGS and the MSFHR. Funding was provided through operating grants from CIHR and NSERC. The authors declare no conflicts of interest in connection with the manuscript.
Author information
Authors and Affiliations
Contributions
HTL and AEL performed the experiments, analysized the results and and wrote the manuscript. HTL prepared figures 12; AEL prepared figures 36; YK contributed to figure 3 and 4; BQ contributed to figure 1; FEN participated in data analysis and reviewed the manuscript; JAG participated in data analysis and wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplmentary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Law, H., Lin, AJ., Kim, Y. et al. Francisella tularensis Uses Cholesterol and Clathrin-Based Endocytic Mechanisms to Invade Hepatocytes. Sci Rep 1, 192 (2011). https://doi.org/10.1038/srep00192
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00192
This article is cited by
-
Flavescence dorée phytoplasma enters insect cells by a clathrin-mediated endocytosis allowing infection of its insect vector
Scientific Reports (2023)
-
Clathrin-dependent internalization, signaling, and metabolic processing of guanylyl cyclase/natriuretic peptide receptor-A
Molecular and Cellular Biochemistry (2018)
-
Invasion and persistence of Mycoplasma bovis in embryonic calf turbinate cells
Veterinary Research (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.