Abstract
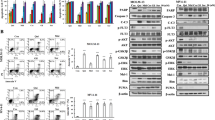
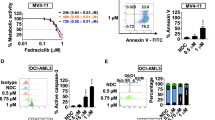
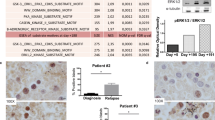
PKC412 is a staurosporine derivative that inhibits several protein kinases including FLT3, and is highly anticipated as a novel therapeutic agent for acute myeloblastic leukemia (AML) carrying FLT3 mutations. In this study, we show that PKC412 exerts differential cell cycle effects on AML cells depending on the presence of FLT3 mutations. PKC412 elicits massive apoptosis without markedly affecting cell cycle patterns in AML cell lines with FLT3 mutations (MV4-11 and MOLM13), whereas it induces G2 arrest but not apoptosis in AML cell lines without FLT3 mutations (THP-1 and U937). In MV4-11 and MOLM13 cells, PKC412 inactivates Myt-1 and activates CDC25c, leading to the activation of CDC2. Activated CDC2 phosphorylates Bad at serine-128 and facilitates its translocation to the mitochondria, where Bad triggers apoptosis. In contrast, PKC412 inactivates CDC2 by inducing serine-216 phosphorylation and subsequent cytoplasmic sequestration of CDC25c in THP-1 and U937 cells. As a result, cells are arrested in the G2 phase of the cell cycle, but do not undergo apoptosis because Bad is not activated. The FLT3 mutation-dependent differential cell cycle effect of PKC412 is considered an important factor when PKC412 is combined with cell cycle-specific anticancer drugs in the treatment of cancer and leukemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Begemann M, Kashimawo SA, Heitjan DF, Schiff PB, Bruce JN, Weinstein IB . (1998). Treatment of human glioblastoma cells with the staurosporine derivative CGP 41251 inhibits CDC2 and CDK2 kinase activity and increases radiation sensitivity. Anticancer Res 18: 2275–2282.
Donovan N, Becker EB, Konishi Y, Bonni A . (2002). JNK phosphorylation and activation of BAD couples the stress-activated signaling pathway to the cell death machinery. J Biol Chem 277: 40944–40949.
Furukawa Y, Vu HA, Akutsu M, Odgerel T, Izumi T, Tsunoda S et al. (2007). Divergent cytotoxic effects of PKC412 in combination with conventional antileukemic agents in FLT3 mutation-positive versus negative leukemia cell lines. Leukemia 21: 1005–1014.
Galaktionov K, Jessus C, Beach D . (1995). Raf1 interaction with Cdc25 phosphatase ties mitogenic signal transduction to cell cycle activation. Genes Dev 9: 1046–1058.
Galonek HL, Hardwick JM . (2006). Upgrading the bcl-2 network. Nat Cell Biol 12: 1317–1319.
Gilliland DG, Griffin JD . (2002). The roles of FLT3 in hematopoiesis and leukemia. Blood 100: 1532–1542.
Green DR . (2006). At the gates of death. Cancer Cell 9: 328–330.
Griffin JD . (2004). FLT3 tyrosine kinase as a target in acute leukemias. Hematol J 5: 188–190.
Ikegami Y, Yano S, Nakao K . (1996). Effects of the new selective protein kinase C inhibitor 4′-N-benzoyl staurosporine on cell cycle distribution and growth inhibition in human small cell lung cancer cells. Arzneimittelforschung 46: 201–204.
Kelly LM, Liu Q, Kutok JL, Williams IR, Boulton CL, Gilliland DG . (2002). FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood 99: 310–318.
Kim KT, Levis M, Small D . (2006). Constitutively activated FLT3 phosphorylates BAD partially through pim-1. Br J Haematol 134: 500–509.
Kiyoi H, Naoe T, Yokota S, Nakao M, Minami S, Kuriyama K et al. (1997). Internal tandem duplication of FLT3 associated with leukocytosis in acute promyelocytic leukemia. Leukemia 11: 1447–1452.
Konishi Y, Lehtinen M, Donovan N, Bonni A . (2002). Cdc2 phosphorylation of BAD Links the cell cycle to the cell death machinery. Mol Cell 9: 1005–1016.
Levis M, Pham R, Smith BD, Small D . (2004). In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood 104: 1145–1150.
Levis M, Small D . (2005). FLT3 tyrosine kinase inhibitors. Int J Hematol 82: 100–107.
Lopez-Girona A, Furnari B, Mondesert O, Russell P . (1999). Nuclear localization of Cdc25 is regulated by DNA damage and a14-3-3 protein. Nature 397: 172–175.
Lyman SD, James L, Vanden Bos T, de Vries P, Brasel K, Gliniak B et al. (1993). Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell 75: 1157–1167.
Neben K, Schnittger S, Bross B, Tews B, Kokocinski F, Haferlach T et al. (2005). Distinct gene expressions patterns associated with FLT3- and NRAS- activating mutations in acute myeloid leukemia with normal karyotype. Oncogene 24: 1580–1588.
Propper DJ, McDonald AC, Man A, Thavasu P, Balkwill F, Braybrooke JP et al. (2001). Phase I and pharmacokinetic study of PKC412, an inhibitor of protein kinase C. J Clin Oncol 19: 1485–1492.
Quentmeier H, Reinhardt J, Zaborski M, Drexler HG . (2003). FLT3 mutations in acute myeloid leukemia cell lines. Leukemia 17: 120–124.
Schmitt E, Beauchemin M, Bertrand R . (2007). Nuclear colocalization and interaction between bcl-xL and cdk1 (cdc2) during G2/M cell-cycle checkpoint. Oncogene 26: 5851–5865.
Small D, Levenstein M, Kim E, Carow C, Amin S, Rockwell P et al. (1994). STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci USA 91: 459–463.
Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD et al. (2005). Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood 105: 54–60.
Watanabe N, Broome M, Hunter T . (1995). Regulation of the human Wee1 CDK tyrosine 15 kinase during cell cycle. EMBO J 14: 1878–1891.
Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T et al. (2002). Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell 1: 433–443.
Yang X, Lui L, Sternberg D, Tang L, Galinsky I, DeAngelo D et al. (2005). The FLT3 internal tandem duplication mutation prevents apoptosis in interleukin 3-deprived BaF3 cells due to protein kinase A and ribosomal S6 Kinase 1-mediated BAD phosphorylation at serine 112. Cancer Res 65: 7338–7347.
Yao Q, Nishiuchi R, Li Q, Kumar AR, Hudson WA, Kersey JH . (2003). FLT3 expressing leukemias are selectively sensitive to inhibitors of the molecular chaperone heat shock protein 90 through destabilization of signal transduction-associated kinases. Clin Cancer Res 9: 4483–4493.
Yee KW, Schittenhelm M, O'Farrell AM, Town AR, McGreevey L, Bainbridge T et al. (2004). Synergistic effect of SU11248 with cytarabine or daunorubicin on FLT3 ITD-positive leukemic cells. Blood 104: 4202–4209.
Zha J, Harada H, Yang F, Jockel J, Korsmeyer SJ . (1996). Serine phosphorylation of death agonist Bad in response to survival factor results in binding to 14-3-3 not BCL-XL. Cell 87: 619–628.
Acknowledgements
This work was supported in part by the High-Tech Research Center Project for Private Universities: Matching Fund Subsidy from MEXT 2002–2006, and grants from the Vehicle Racing Commemorative Foundation and Sankyo Foundation of Life Science (to YF). JK is a winner of the Jichi Medical School Young Investigator Award. TW is a winner of the Jichi Medical School Graduate Student Award.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Odgerel, T., Kikuchi, J., Wada, T. et al. The FLT3 inhibitor PKC412 exerts differential cell cycle effects on leukemic cells depending on the presence of FLT3 mutations. Oncogene 27, 3102–3110 (2008). https://doi.org/10.1038/sj.onc.1210980
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210980
Keywords
This article is cited by
-
Which FLT3 Inhibitor for Treatment of AML?
Current Treatment Options in Oncology (2022)
-
MYC-dependent downregulation of telomerase by FLT3 inhibitors is required for their therapeutic efficacy on acute myeloid leukemia
Annals of Hematology (2018)
-
Activation of HIV-1 expression in latently infected CD4+ T cells by the small molecule PKC412
Virology Journal (2016)
-
BPR1J-097, a novel FLT3 kinase inhibitor, exerts potent inhibitory activity against AML
British Journal of Cancer (2012)
-
Bortezomib overcomes cell adhesion-mediated drug resistance through downregulation of VLA-4 expression in multiple myeloma
Oncogene (2009)