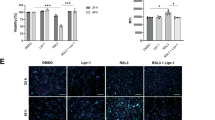
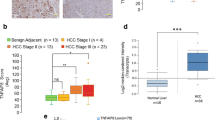
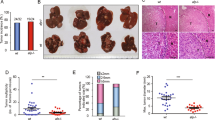
Abstract
BRE binds to the cytoplasmic domains of tumor necrosis factor receptor-1 and Fas, and in cell lines can attenuate death receptor-initiated apoptosis by inhibiting t-BID-induced activation of the mitochondrial apoptotic pathway. Overexpression of BRE by transfection can also attenuate intrinsic apoptosis and promote growth of the transfected Lewis lung carcinoma line in mice. There is, however, a complete lack of in vivo data about the protein. Here, we report that by using our BRE-specific monoclonal antibody on the immunohistochemistry of 123 specimens of human hepatocellular carcinoma (HCC), significant differences in BRE expression levels between the paired tumoral and non-tumoral regions (P<2.2e−16) were found. Marked overexpression of BRE was detected in majority of the tumors, whereas most non-tumoral regions expressed the same low level of the protein as in normal livers. To investigate whether BRE overexpression could promote cell survival in vivo, liver-specific transgenic BRE mice were generated and found to be significantly resistant to Fas-mediated lethal hepatic apoptosis. The transgenic model also revealed post-transcriptional regulation of Bre level in the liver, which was not observed in HCC and non-HCC cell lines. Indeed, all cell lines analysed express high levels of BRE. In conclusion, BRE is antiapoptotic in vivo, and may promote tumorigenesis when overexpressed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Aggarwal BB . (2003). Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3: 745–756.
Bai L, Ni HM, Chen X, DiFrancesca D, Yin XM . (2005). Deletion of Bid impedes cell proliferation and hepatic carcinogenesis. Am J Pathol 166: 1523–1532.
Barber GN . (2001). Host defense, viruses and apoptosis. Cell Death Differ 8: 113–126.
Chan BC, Li Q, Chow SK, Ching AK, Liew CT, Lim PL et al. (2005). BRE enhances in vivo growth of tumor cells. Biochem Biophys Res Commun 326: 268–273.
Chan BC, To KF, Pang JC, Chung YF, Lo KW, Tong JH et al. (2002). Generation of monoclonal antibodies against Hong Kong nasopharyngeal carcinoma-associated Epstein–Barr virus latent membrane protein 1 (LMP1). Int J Cancer 102: 492–498.
Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T et al. (2001). BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell 8: 705–711.
Ching AK, Li PS, Chan WY, Ma CH, Lee SS, Lim PL et al. (2000). Strand bias in Ig somatic hypermutation is determined by signal sequence within the variable region. Int Immunol 12: 1245–1253.
Cory S, Huang DC, Adams JM . (2003). The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22: 8590–8607.
de la Coste A, Fabre M, McDonell N, Porteu A, Gilgenkrantz H, Perret C et al. (1999a). Differential protective effects of Bcl-xL and Bcl-2 on apoptotic liver injury in transgenic mice. Am J Physiol 277: G702–G708.
de La Coste A, Mignon A, Fabre M, Gilbert E, Porteu A, Van Dyke T et al. (1999b). Paradoxical inhibition of c-myc-induced carcinogenesis by Bcl-2 in transgenic mice. Cancer Res 59: 5017–5022.
Dive C, Evans CA, Whetton AD . (1992). Induction of apoptosis—new targets for cancer chemotherapy. Semin Cancer Biol 3: 417–427.
Dong Y, Hakimi MA, Chen X, Kumaraswamy E, Cooch NS, Godwin AK et al. (2003). Regulation of BRCC, a holoenzyme complex containing BRCA1 and BRCA2, by a signalosome-like subunit and its role in DNA repair. Mol Cell 12: 1087–1099.
Engels IH, Stepczynska A, Stroh C, Lauber K, Berg C, Schwenzer R et al. (2000). Caspase-8/FLICE functions as an executioner caspase in anticancer drug-induced apoptosis. Oncogene 19: 4563–4573.
Green DR, Reed JC . (1998). Mitochondria and apoptosis. Science 281: 1309–1312.
Gross A, McDonnell JM, Korsmeyer SJ . (1999). BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13: 1899–1911.
Gu C, Castellino A, Chan JY, Chao MV . (1998). BRE: a modulator of TNF-alpha action. FASEB J 12: 1101–1118.
Huang DC, O’Reilly LA, Strasser A, Cory S . (1997). The anti-apoptosis function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry. EMBO J 16: 4628–4638.
Kohler G, Milstein C . (1975). Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256: 495–497.
Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH . (2000). Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 7: 1166–1173.
Lacronique V, Mignon A, Fabre M, Viollet B, Rouquet N, Molina T et al. (1996). Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat Med 2: 80–86.
Li L, Yoo H, Becker FF, Ali-Osman F, Chan JY . (1995). Identification of a brain- and reproductive-organs-specific gene responsive to DNA damage and retinoic acid. Biochem Biophys Res Commun 206: 764–774.
Li Q, Ching AK, Chan BC, Chow SK, Lim PL, Ho TC et al. (2004). A death receptor-associated anti-apoptotic protein, BRE, inhibits mitochondrial apoptotic pathway. J Biol Chem 279: 52106–52116.
Lowe SW, Cepero E, Evan G . (2004). Intrinsic tumour suppression. Nature 432: 307–315.
Nagata S . (1997). Apoptosis by death factor. Cell 88: 355–365.
Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y et al. (1993). Lethal effect of the anti-Fas antibody in mice. Nature 364: 806–809.
O’Reilly LA, Huang DC, Strasser A . (1996). The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO J 15: 6979–6990.
Pierce RH, Vail ME, Ralph L, Campbell JS, Fausto N . (2002). Bcl-2 expression inhibits liver carcinogenesis and delays the development of proliferating foci. Am J Pathol 160: 1555–1560.
Rausa FM, Tan Y, Zhou H, Yoo KW, Stolz DB, Watkins SC et al. (2000). Elevated levels of hepatocyte nuclear factor 3beta in mouse hepatocytes influence expression of genes involved in bile acid and glucose homeostasis. Mol Cell Biol 20: 8264–8282.
Rodriguez I, Matsuura K, Khatib K, Reed JC, Nagata S, Vassalli P . (1996). A bcl-2 transgene expressed in hepatocytes protects mice from fulminant liver destruction but not from rapid death induced by anti-Fas antibody injection. J Exp Med 183: 1031–1036.
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ et al. (1998). Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17: 1675–1687.
Strasser A, Harris AW, Huang DC, Krammer PH, Cory S . (1995). Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J 14: 6136–6147.
Tang MK, Wang CM, Shan SW, Chui YL, Ching AK, Chow PH et al. (2006). Comparative proteomic analysis reveals a function of the novel death receptor-associated protein BRE in the regulation of prohibitin and p53 expression and proliferation. Proteomics 6: 2376–2385.
Vail ME, Pierce RH, Fausto N . (2001). Bcl-2 delays and alters hepatic carcinogenesis induced by transforming growth factor alpha. Cancer Res 61: 594–601.
Vaux DL, Korsmeyer SJ . (1999). Cell death in development. Cell 96: 245–254.
Yan C, Costa RH, Darnell Jr JE, Chen JD, Van Dyke TA . (1990). Distinct positive and negative elements control the limited hepatocyte and choroid plexus expression of transthyretin in transgenic mice. EMBO J 9: 869–878.
Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B et al. (1999). Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400: 886–891.
Yu J, Hui AY, Chu ES, Cheng AS, Go MY, Chan HL et al. (2007). Expression of a COX-2 transgene in murine liver causes hepatitis. Gut 56: 991–999.
Zamzami N, Brenner C, Marzo I, Susin SA, Kroemer G . (1998). Subcellular and submitochondrial mode of action of Bcl-2-like oncoproteins. Oncogene 16: 2265–2282.
Zhivotovsky B, Orrenius S . (2006). Carcinogenesis and apoptosis: paradigms and paradoxes. Carcinogenesis 27: 1939–1945.
Acknowledgements
We gratefully acknowledge Ms Peggy Hoi-Ying Fung for clerical help in preparing this paper. This work was supported by a direct grant for research of Project Code 2041117 from the Hong Kong Research Grants Council Direct Allocation to the Chinese University of Hong Kong, and partially by an Earmarked Grant CUHK 4421/03M from the Research Grant Committee of Hong Kong.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Chan, BL., Ching, AK., To, KF. et al. BRE is an antiapoptotic protein in vivo and overexpressed in human hepatocellular carcinoma. Oncogene 27, 1208–1217 (2008). https://doi.org/10.1038/sj.onc.1210733
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210733
Keywords
This article is cited by
-
SAMD13 serves as a useful prognostic biomarker for hepatocellular carcinoma
European Journal of Medical Research (2023)
-
Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: an insight
Cancer Cell International (2018)
-
C-terminal BRE overexpression in 11q23-rearranged and t(8;16) acute myeloid leukemia is caused by intragenic transcription initiation
Leukemia (2018)
-
Macrolide antibiotics differentially influence human HepG2 cytotoxicity and modulate intrinsic/extrinsic apoptotic pathways in rat hepatocellular carcinoma model
Naunyn-Schmiedeberg's Archives of Pharmacology (2017)
-
Over-expression of the long non-coding RNA HOTTIP inhibits glioma cell growth by BRE
Journal of Experimental & Clinical Cancer Research (2016)