Abstract
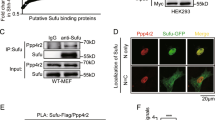
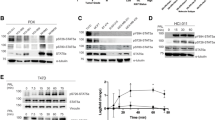
Signal transducer and activator of transcription 3 (Stat3) belongs to a family of latent cytoplasmic transcription factors important for cytokine signaling. Stat3 is constitutively activated in various tumors, and activated Stat3 itself also acts as an oncogene. Transcriptional activity of Stat3 is controlled by Tyr-phosphorylation, followed by dimerization and nuclear translocation. However, phosphorylation on Ser727 is indispensable for its maximal transcriptional activity with unclear mechanism. Here, we report that peptidyl-prolyl cis/trans isomerase 1 (Pin1), which specifically recognizes the pSer/Thr-Pro motifs on its target proteins, interacts with Stat3 upon cytokine/growth factor stimulation. Overexpression of Pin1 promotes Stat3 transcriptional activity and target gene expression, as well as recruitment of transcription coactivator, p300. These effects, however, were compromised in the Pin1-deficient cells, and were totally dependent on the Ser727 phosphorylation site. Finally, we showed that Pin1 enhances Stat3-mediated epithelial–mesenchymal transition in breast cancer cells induced by oncostatin M. Our data reveal a novel, Ser727 phosphorylation-dependent, post-translational regulation mechanism for Stat3.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bao L, Kimzey A, Sauter G, Sowadski JM, Lu KP, Wang DG . (2004). Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am J Pathol 164: 1727–1737.
Bowman T, Garcia R, Turkson J, Jove R . (2000). STATs in oncogenesis. Oncogene 19: 2474–2488.
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C et al. (1999). Stat3 as an oncogene. Cell 98: 295–303.
Cao X, Tay A, Guy GR, Tan YH . (1996). Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol 16: 1595–1603.
Clevenger CV . (2004). Roles and regulation of stat family transcription factors in human breast cancer. Am J Pathol 165: 1449–1460.
Crenshaw DG, Yang J, Means AR, Kornbluth S . (1998). The mitotic peptidyl-prolyl isomerase, Pin1, interacts with Cdc25 and Plx1. EMBO J 17: 1315–1327.
Danial NN, Pernis A, Rothman PB . (1995). Jak–STAT signaling induced by the v-abl oncogene. Science 269: 1875–1877.
Darnell Jr JE, Kerr IM, Stark GR . (1994). Jak–STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264: 1415–1421.
Darnell Jr JE . (1997). STATs and gene regulation. Science 277: 1630–1635.
Decker T, Kovarik P . (2000). Serine phosphorylation of STATs. Oncogene 19: 2628–2637.
Fujimori F, Takahashi K, Uchida C, Uchida T . (1999). Mice lacking Pin1 develop normally, but are defective in entering cell cycle from G(0) arrest. Biochem Biophys Res Commun 265: 658–663.
Holzer RG, Ryan RE, Tommack M, Schlekeway E, Jorcyk CL . (2004). Oncostatin M stimulates the detachment of a reservoir of invasive mammary carcinoma cells: role of cyclooxygenase-2. Clin Exp Metastasis 21: 167–176.
Jorcyk CL, Holzer RG, Ryan RE . (2006). Oncostatin M induces cell detachment and enhances the metastatic capacity of T-47D human breast carcinoma cells. Cytokine 33: 323–336.
Levy DE, Lee CK . (2002). What does Stat3 do? J Clin Invest 109: 1143–1148.
Lim CP, Cao X . (1999). Serine phosphorylation and negative regulation of Stat3 by JNK. J Biol Chem 274: 31055–31061.
Lu KP, Hanes SD, Hunter T . (1996). A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature 380: 544–547.
Lu PJ, Zhou XZ, Shen M, Lu KP . (1999). Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science 283: 1325–1328.
Lufei C, Ma J, Huang G, Zhang T, Novotny-Diermayr V, Ong CT et al. (2003). GRIM-19, a death-regulatory gene product, suppresses Stat3 activity via functional interaction. EMBO J 22: 1325–1335.
Ng DC, Lin BH, Lim CP, Huang G, Zhang T, Poli V et al. (2006). Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J Cell Biol 172: 245–257.
Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H et al. (1999). Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J 18: 4657–4668.
Thiery JP . (2003). Epithelial–mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 15: 740–746.
Wen Z, Zhong Z, Darnell Jr JE . (1995). Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82: 241–250.
Winkler KE, Swenson KI, Kornbluth S, Means AR . (2000). Requirement of the prolyl isomerase Pin1 for the replication checkpoint. Science 287: 1644–1647.
Wulf G, Finn G, Suizu F, Lu KP . (2005). Phosphorylation-specific prolyl isomerization: is there an underlying theme? Nat Cell Biol 7: 435–441.
Wulf G, Garg P, Liou YC, Iglehart D, Lu KP . (2004). Modeling breast cancer in vivo and ex vivo reveals an essential role of Pin1 in tumorigenesis. EMBO J 23: 3397–3407.
Wulf G, Ryo A, Liou YC, Lu KP . (2003). The prolyl isomerase Pin1 in breast development and cancer. Breast Cancer Res 5: 76–82.
Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J et al. (1995). Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science 269: 81–83.
Zacchi P, Gostissa M, Uchida T, Salvagno C, Avolio F, Volinia S et al. (2002). The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature 419: 853–857.
Zhang F, Li C, Halfter H, Liu J . (2003). Delineating an oncostatin M-activated STAT3 signaling pathway that coordinates the expression of genes involved in cell cycle regulation and extracellular matrix deposition of MCF-7 cells. Oncogene 22: 894–905.
Zhang T, Seow KT, Ong CT, Cao X . (2002). Interdomain interaction of Stat3 regulates its Src homology 2 domain-mediated receptor binding activity. J Biol Chem 277: 17556–17563.
Zheng H, You H, Zhou XZ, Murray SA, Uchida T, Wulf G et al. (2002). The prolyl isomerase Pin1 is a regulator of p53 in genotoxic response. Nature 419: 849–853.
Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G et al. (2000). Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell 6: 873–883.
Acknowledgements
This work was supported by the Agency for Science, Technology and Research of Singapore. T Uchida is supported by Grant-in-Aid for Scientific Research on Priority Areas of Japan. X Cao is an adjunct staff in the Department of Biochemistry, National University of Singapore.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Lufei, C., Koh, T., Uchida, T. et al. Pin1 is required for the Ser727 phosphorylation-dependent Stat3 activity. Oncogene 26, 7656–7664 (2007). https://doi.org/10.1038/sj.onc.1210567
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210567
Keywords
This article is cited by
-
BUB1 drives the occurrence and development of bladder cancer by mediating the STAT3 signaling pathway
Journal of Experimental & Clinical Cancer Research (2021)
-
The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers
Scientific Reports (2020)
-
Prolyl isomerase Pin1: a promoter of cancer and a target for therapy
Cell Death & Disease (2018)
-
PIN1 in breast development and cancer: a clinical perspective
Cell Death & Differentiation (2017)
-
The isomerase PIN1 controls numerous cancer-driving pathways and is a unique drug target
Nature Reviews Cancer (2016)