Abstract
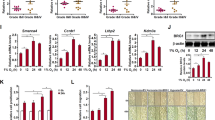
The SWI/SNF chromatin-remodeling complex serves as a master switch that directs and limits the execution of specific cellular programs, such as differentiation and growth control. SWI/SNF function requires one of two paralogous ATPase subunits, Brahma (BRM) or BRM-related gene 1 (BRG1), which we previously found are lost together in cancer cell lines and primary lung cancers. Although BRG1 has been found to be mutated in cancer cell lines, the mechanisms underlying BRM silencing are not known. To address this question, we sequenced BRM in 10 BRM/BRG1-deficient cancer cell lines and found that BRM was devoid of abrogating mutations. Moreover, histone deacetylase (HDAC) inhibitors restored BRM expression in each of these BRG1/BRM-deficient cancer cell lines, indicating that epigenetic silencing is a major mechanism underlying the loss of BRM expression. Despite their ability to restore BRM expression, these HDAC inhibitors also blocked BRM function when present. However, after their removal, we observed that BRM expression remained elevated for several days, and during this period, BRM activity was detected. We also found that the suppression of BRM occurs in a broad range of human tumor types and that loss of one or both BRM alleles potentiated tumor development in mice. Thus, BRG1 and BRM are silenced by different mechanisms, and it may be possible to clinically target and reexpress BRM in a number of tumor types, potentially impacting tumor development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bourachot B, Yaniv M, Muchardt C . (2003). Growth inhibition by the mammalian SWI-SNF subunit Brm is regulated by acetylation. EMBO J 22: 6505–6515.
Coisy-Quivy M, Disson O, Roure V, Muchardt C, Blanchard JM, Dantonel JC . (2006). Role for brm in cell growth control. Cancer Res 66: 5069–5076.
Fukuoka J, Fujii T, Shih JH, Dracheva T, Meerzaman D, Player A et al. (2004). Chromatin remodeling factors and BRM/BRG1 expression as prognostic indicators in non-small cell lung cancer. Clin Cancer Res 10: 4314–4324.
Khavari PA, Peterson CL, Tamkun JW, Mendel DB, Crabtree GR . (1993). BRG1 contains a conserved domain of the SWI2/SNF2 family necessary for normal mitotic growth and transcription. Nature 366: 170–174.
Klochendler-Yeivin A, Muchardt C, Yaniv M . (2002). SWI/SNF chromatin remodeling and cancer. Curr Opin Genet Dev 12: 73–79.
Machida Y, Murai K, Miyake K, Iijima S . (2001). Expression of chromatin remodeling factors during neural differentiation. J Biochem (Tokyo) 129: 43–49.
Muchardt C, Yaniv M . (1993). A human homologue of Saccharomyces cerevisiae SNF2/SWI2 and Drosophila brm genes potentiates transcriptional activation by the glucocorticoid receptor. EMBO J 12: 4279–4290.
Muchardt C, Yaniv M . (2001). When the SWI/SNF complex remodels – the cell cycle. Oncogene 20: 3067–3075.
Reisman DN, Sciarrotta J, Wang W, Funkhouser WK, Weissman BE . (2003). Loss of BRG1/BRM in human lung cancer cell lines and primary lung cancers: correlation with poor prognosis. Cancer Res 63: 560–566.
Reisman DN, Strobeck MW, Betz BL, Sciariotta J, Funkhouser Jr W, Murchardt C et al. (2002). Concomitant down-regulation of BRM and BRG1 in human tumor cell lines: differential effects on RB-mediated growth arrest vs CD44 expression. Oncogene 21: 1196–1207.
Roberts CW, Orkin SH . (2004). The SWI/SNF complex – chromatin and cancer. Nat Rev Cancer 4: 133–142.
Simone C . (2006). SWI/SNF: the crossroads where extracellular signaling pathways meet chromatin. J Cell Physiol 207: 309–314.
Strobeck MW, DeCristofaro MF, Banine F, Weissman BE, Sherman LS, Knudsen ES . (2001). The BRG-1 subunit of the SWI/SNF complex regulates CD44 expression. J Biol Chem 276: 9273–9278.
Strobeck MW, Reisman DN, Gunawardena RW, Betz BL, Angus SP, Knudsen KE et al. (2002). Compensation of BRG-1 function by Brm: insight into the role of the core SWI/SNF subunits in RB-signaling. J Biol Chem 21: 21.
Sudarsanam P, Iyer VR, Brown PO, Winston F . (2000). Whole-genome expression analysis of snf/swi mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci USA 97: 3364–3369.
Trotter KW, Archer TK . (2004). Reconstitution of glucocorticoid receptor-dependent transcription in vivo. Mol Cell Biol 24: 3347–3358.
Tuveson DA, Jacks T . (1999). Modeling human lung cancer in mice: similarities and shortcomings. Oncogene 18: 5318–5324.
Wang W, Xue Y, Zhou S, Kuo A, Cairns BR, Crabtree GR . (1996). Diversity and specialization of mammalian SWI/SNF complexes. Genes Dev 10: 2117–2130.
Wong AK, Shanahan F, Chen Y, Lian L, Ha P, Hendricks K et al. (2000). BRG1, a component of the SWI-SNF complex, is mutated in multiple human tumor cell lines. Cancer Res 60: 6171–6177.
Yamamichi N, Yamamichi-Nishina M, Mizutani T, Watanabe H, Minoguchi S, Kobayashi N et al. (2005). The Brm gene suppressed at the post-transcriptional level in various human cell lines is inducible by transient HDAC inhibitor treatment, which exhibits antioncogenic potential. Oncogene 24: 5471–5481.
Acknowledgements
We thank Rachel Dresbeck for her diligent efforts in editing this manuscript. This work is supported in part by University of Michigan Diabetes Core grant DK20572, University of Michigan Cancer Center grant 5-P30-CA46592 and K08 grant CA092149–03. Christian Murchardt has received grant support from ‘Canceropole IdF’. Thanks to Drs Aubrey Thompson and Thomas Carey for their critical feedback on this manuscript. We also thank Drs Kleer, Tom Giordano, David Beer, Rajal Shah and Kathy Cho for their gift of the breast, colon, esophageal, bladder and ovarian TMAs respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Glaros, S., Cirrincione, G., Muchardt, C. et al. The reversible epigenetic silencing of BRM: implications for clinical targeted therapy. Oncogene 26, 7058–7066 (2007). https://doi.org/10.1038/sj.onc.1210514
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210514
Keywords
This article is cited by
-
Multi-omic alterations of the SWI/SNF complex define a clinical subgroup in lung adenocarcinoma
Clinical Epigenetics (2022)
-
Exploiting vulnerabilities of SWI/SNF chromatin remodelling complexes for cancer therapy
Oncogene (2021)
-
SMARCA4/2 loss inhibits chemotherapy-induced apoptosis by restricting IP3R3-mediated Ca2+ flux to mitochondria
Nature Communications (2021)
-
BRM: the core ATPase subunit of SWI/SNF chromatin-remodelling complex—a tumour suppressor or tumour-promoting factor?
Epigenetics & Chromatin (2019)
-
SMARCA2-deficiency confers sensitivity to targeted inhibition of SMARCA4 in esophageal squamous cell carcinoma cell lines
Scientific Reports (2019)