Abstract
Preclinical studies have shown that stress and glucocorticoids increase mesolimbic dopamine (DA) and thereby facilitate psychostimulant self-administration. The relationship between stress-induced cortisol and mesolimbic DA responses to psychostimulants has not been studied in humans. To test the hypotheses that glucocorticoid responses to psychological stress are correlated with DA and subjective responses to psychostimulants in humans, 25 healthy adults (18–29 years) completed the Trier Social Stress Test (TSST) and two positron emission tomography (PET) scans with high-specific [11C]raclopride. The first scan was preceded by intravenous saline and the second by amphetamine (AMPH). Findings showed that stress-induced cortisol levels were positively associated with AMPH-induced DA release in the ventral striatum and other striatal regions. Subjects with higher cortisol responses to stress also reported more positive subjective drug effects with AMPH than subjects with lower responses. The results are consistent with preclinical findings showing an interrelationship between glucocorticoids and mesolimbic DA dynamics, which may influence psychostimulant self-administration in humans.
Similar content being viewed by others
INTRODUCTION
A large body of evidence from preclinical studies suggests that the rewarding effects of drugs of abuse are derived from their ability to alter mesocorticolimbic dopamine (DA) neurotransmission (Bonci et al, 2003). A region within the ventral striatum, the nucleus accumbens, is a particularly important substrate for DA effects. Findings from preclinical studies have shown that psychostimulants, opioids, and alcohol all increase synaptic DA accumulation within this important brain region (Wise, 1998).
In the past decade, the use of positron emission tomography (PET) imaging has facilitated advancements in the field of substance abuse research by showing that certain observations originally made in rodent models can be translated to the human condition. Specifically, PET imaging has demonstrated that amphetamine (AMPH), methylphenidate, and cocaine increase mesolimbic DA concentrations and that the magnitude of DA responsiveness to these drugs of abuse correlates with their positive subjective effects (Oswald et al, 2005; Volkow et al, 2004; Martinez et al, 2004; Leyton et al, 2002). Given that the mesolimbic system is an important mediator of drug reward, it is important to understand the environmental and genetic factors that modulate its function. In this regard, the stress response has become a point of focus for increasing our understanding of the neurobiological processes that underlie individual vulnerability for addiction.
In humans, stress has been implicated as an etiological factor in the development substance use disorders (Gordon, 2002) as well as an important precipitant of relapse (Breese et al, 2005). The glucocorticoid response to stress is a principal biological adaptation to adversity. Stress provokes CRH release from the hypothalamus, which in turn stimulates pituitary ACTH secretion. Subsequently, ACTH induces adrenal secretion of glucocorticoids, specifically corticosterone in rodents and cortisol in humans. The magnitude of the cortisol response to stress is thought to be regulated by the interaction of environmental and genetic determinants (Federenko et al, 2004).
A large body of preclinical research has now been devoted to the interactions between stress, glucocorticoids, and mesolimbic DA neurons and their involvement in the pathophysiological mechanisms of drug abuse (Piazza et al, 1996). Stress-induced LTP can be blocked with a glucocorticoid receptor (GR) antagonist (Saal et al, 2003). Using microdialysis techniques, investigators have shown that an array of stress paradigms increases mesocorticolimbic DA activity and that these effects are accompanied by behavioral manifestations (Cadoni et al, 2003; Cuadra et al, 2001). Glucocorticoid administration facilitates the psychomotor stimulant effects of cocaine (Marinelli et al, 1994; Cador et al, 1993) and morphine (Marinelli et al, 1994) in rodents. A variety of stress paradigms have been shown to increase stimulant self-administration (Piazza et al, 1996). In contrast, adrenalectomy abrogates cocaine self-administration (Goeders et al, 1998). Deroche-Gamonet et al (2003) recently corroborated these findings by utilizing the Cre/LoxP system to create mice with a targeted disruption of GRs, showing that the knockout mice had a dramatic decrease in cocaine self-administration. In human subjects, greater stress-induced cocaine craving has been associated with a shorter time to cocaine relapse (Sinha et al, 2003). Moreover, stress-induced corticotropin and cortisol responses predicted higher amounts of cocaine use per occasion in the 90-day follow-up (Sinha et al, 2006).
We previously demonstrated during PET imaging in humans that AMPH-induced DA release is correlated with AMPH-induced cortisol secretion as well as with positive subjective drug responses including drug ‘liking,’ ‘desire,’ ‘good effect,’ ‘high,’ and ‘rush’ (Oswald et al, 2005). However, the prior study did not clarify whether cortisol responses to a psychological stressor would also be associated with DA responses to AMPH. Based on our prior observations, we posited that individuals with greater cortisol responses to stress would have greater DA release and more positive subjective responses to AMPH than persons with lower stress reactivity. This hypothesis is consistent with findings from preclinical studies showing that stress cross-sensitizes with both psychostimulants (Kosten et al, 2003) and alcohol (Yavich and Tiihonen, 2000) leading to greater increases in striatal DA concentrations following drug administration.
METHODS
Screening Procedures
Twenty-five healthy males (n=17) and females (n=8) of European ancestry, aged 18–29 years, were recruited for study participation by newspaper advertisements and fliers posted in Baltimore area communities. All participants provided written informed consent under the oversight of The Johns Hopkins School of Medicine Institutional Review Board. Sixteen of the subjects were included in our first PET study that examined the relationship between AMPH-induced cortisol vs AMPH-induced DA release (Oswald et al, 2005). Subject assessment included a medical history and physical exam performed by a physician, complete blood count, comprehensive metabolic panel (including renal and hepatic function tests), electrocardiogram, urinalysis, alcohol breathalyzer test, and urine toxicology screen. Master's-level interviewers administered the Semi-Structured Assessment for the Genetics of Alcoholism (Bucholz et al, 1994) to identify DSM-IV Axis I psychiatric diagnoses, including alcohol/drug abuse or dependence.
Exclusion criteria included (a) presence of a lifetime DSM-IV Axis I disorder; (b) treatment in the last 6 months with antidepressants, neuroleptics, sedative hypnotics, psychostimulants, glucocorticoids, appetite suppressants, estrogens, opiate, or DA medications; (c) use of any prescription medications within the past 30 days; (d) women currently using a hormonal method of birth control or hormone replacement therapy, or currently pregnant or lactating; (e) an active medical condition; (f) report drinking more than 50 alcoholic drinks per month or illicit drug use within the 90 days before participation; and (g) current smokers. For women, the Trier Stress Test and PET were conducted during the follicular phase of the menstrual cycle determined by progesterone levels drawn on the day of the session. Women with progesterone levels <1 ng/ml were identified as being in the follicular phase of the menstrual cycle.
MRI Assessment and Mask Fitting
An spoiled gradient sequence (SPGR) MRI volume was acquired as 124 transaxial images for anatomical identification of brain structures using the following parameters: repetition time, 35 ms; echo time, 6 ms; flip angle, 45°; slice thickness, 1.5 mm with no gap; field of view, 24 × 18 cm2; and image acquisition matrix, 256 × 192, reformatted to 256 × 256. In addition, a double echo MRI volume was obtained for screening subjects for incidental cerebral abnormalities. The scanning conditions were as follows: repetition time, 4000 ms; echo time, 105 ms; flip angle, 90°; slice thickness, 5 with 5 mm gap; field of view, 24 × 18 cm; image acquisition matrix, 256 × 192, reformatted to 256 × 256. To minimize head motion during MRI acquisition and PET scanning, a thermoplastic mask was molded for each subject.
PET Procedures and Data Acquisition
Subjects were admitted to The Johns Hopkins Hospital Outpatient General Clinical Research Center (GCRC) the day before the PET procedures. They were instructed not to ingest any alcohol, drugs, or over-the-counter medications for 48 h before admission. Laboratory studies upon admission included a urine toxicology screen, alcohol breathalyzer test, hematocrit, electrolyte panel, and urine pregnancy screen for women. A calorie-controlled, caffeine-free breakfast was provided to subjects before the PET procedures. Beginning at 0830 hours, subjects underwent two consecutive 90-min PET scans with [11C]raclopride. This radioligand is a benzamide antagonist at the D2 and D3 receptors, previously shown to be sensitive to stimulant-induced changes in brain DA concentration (Oswald et al, 2005). A high-specific activity intravenous (i.v.) bolus injection of approximately 18 mCi [11C]raclopride was administered at the beginning of each scan. Subjects lay supine on the scanner table in a nonspecific baseline condition with their heads restricted with the thermoplastic mask. The first scan was preceded at −5 min by an i.v. injection of saline; the second scan was preceded at −5 min by 0.3 mg/kg AMPH, each delivered over 3 min. The scanning image protocol consisted of up to 30 scan acquisitions in 3D mode, starting from 15-s duration and increasing to 6 min in length over a 90-min period. All images were acquired on the 3D GE Advance whole body PET scanner (GE Medical Systems, Waukesha, WI, USA) and were preceded by a 10-min attenuation scan employing a rotating germanium-68 source. Each PET frame was reconstructed to 35 transaxial images of 128 × 128 matrices by a back-projection algorithm using the manufacturer-provided software and correcting for attenuation, scatter, and dead time. PET frames were co-registered to the frame taken at 20 min by means of the mutual information theory as implemented in SPM2 to reduce head motions between frames (Martinez et al, 2003).
Visual analog rating scales were administered 15 min before and 3, 6, 10, 15, 25, 55, and 85 min after saline and AMPH administration. Subjects rated verbally, on a 5-point scale (0=least, 4=most), the degree to which they were experiencing each of 10 possible drug effects. Positive effects included ‘high,’ ‘rush,’ ‘good effects,’ ‘liking,’ and ‘desire for drug.’ Negative effects included ‘fidgety,’ ‘anxious,’ ‘dizziness,’ ‘dry mouth,’ and ‘distrust’ (Kuwabara et al, 2004).
Subjects were instructed to rest with their eyes closed during the scans. They were permitted to rise briefly after the first scan and were repositioned on the scanner table for the second. All subjects were under continuous cardiovascular monitoring during the scans. Subjects were escorted back to the GCRC following the scans and evaluated by a physician before discharge from the unit.
Volumes of interest and modeling of PET outcome measures were conducted as described previously (Oswald et al, 2005). The percent change in binding potential (BP) from baseline (ie the saline scan) to the AMPH scan was used to estimate DA release as [(BPsaline−BPamphetamine)/BPsaline] × 100, with lower BP values during the AMPH scan indicating greater levels of endogenous DA. It should be noted that although ‘DA release’ is the term that is often used in the PET literature to describe AMPH-induced changes in [11C]-raclopride BP (Endres et al, 1997) increases in DA concentrations that occur following AMPH administration probably result from several different mechanisms, including DA reuptake blockade, reverse transport of DA through the DA transporter (Schmitz et al, 2001), as well as possible actions on endogenous opioid systems (Schad et al, 2002). It is not known to what degree BP changes reflect DA concentration changes or affinity of D2/D3 receptors for DA. The term ‘DA release’ in this paper is employed as a expedient manner of describing changes in [11C]raclopride BP and DA receptor occupancy from the placebo to the AMPH scan with the understanding that these caveats apply to its interpretation.
Trier Social Stress Test
During two separate sessions, subjects reported to the GCRC to complete the passive and active Trier Social Stress Test (TSST). The passive TSST always preceded the active TSST by 3–7 days and was employed to reduce anticipatory anxiety related to the procedure and thus maximize obtaining non-stressed baseline cortisol measurements on the day of the active TSST. Both forms of the TSST were randomized up to a month before or after the PET.
For the active TSST (modified from Kirschbaum et al, 1993), subjects arrived at the GCRC fasting at 0900; a fixed 500 cal meal was consumed after arrival. An i.v. catheter was inserted into a forearm vein at 1200. Subjects rested in a quiet setting until baseline blood samples for cortisol were obtained at 1300, 1315, and 1330 hours. Subjects then listened to audiotaped instructions for the speech and mental arithmetic tasks (Kirschbaum et al, 1993). In these instructions, they were told that they would be taking on the role of a job applicant for the position of hospital administrator. They were given 10 min to prepare a 5-min free speech to ‘sell themselves’ to a group of Johns Hopkins Hospital staff managers who were in another room waiting to interview them. They were told that the speech would be videotaped, that each manager was specially trained to monitor nonverbal behavior, that a voice frequency analysis of nonverbal behavior would be performed, that the speech would be critiqued on content and style, and that verbal pauses and poor eye contact would jeopardize their score. They were also told that following the interview, they would be asked to complete an oral arithmetic challenge that would be judged on speed and accuracy.
Following the preparation time, subjects were escorted to another room where two research staff posing as staff managers were waiting to interview them. Subjects were instructed to stand at the end of a long table with the managers sitting at the other end. One of the managers asked the subject to describe his/her qualifications for hospital administrator, whereas another began operating the video camera. An egg timer sitting prominently on the table was set to keep time. Subjects were expected to utilize the entire 5 min for the speech as described by Kirschbaum et al (1993). At the completion of the speech, subjects were told that the 5 min mental arithmetic task would begin, and the timer was reset. They were then asked to serially subtract 13 from 2322. The managers responded to any mistakes by instructing the subject to ‘Start from the top. Subtract 13 from 2322.’ Subjects were then escorted back to the original room where five additional cortisol specimens were collected at 15 min intervals. The State-Trait Anxiety Inventory (STAI; Spielberger, 1983), was administered before the psychological stressor at 1245 and after the stressor at 1355. All subjects were debriefed about the procedure before leaving. They were informed that the video camera did not contain film, that no voice analysis would be conducted, and that the interviewers were actually persons involved with the study.
The passive TSST was conducted in the same manner as the active Trier except the tape recording informed subjects that they could sit quietly or read for the remainder of the session as blood samples were periodically drawn.
The STAI is a 40-item self-rating scale that measures state (temporal feelings based on situational state) and trait (general anxiety levels or proneness) anxiety. The state scale consists of 20 statements that evaluate how respondents feel ‘right now.’ Respondents describe the intensity of their feelings on a 4-point scale that ranges from ‘not at all’ to ‘very much so.’ The trait scale consists of 20 statements that assess how people generally feel. Respondents are instructed to rate the general frequency of their feelings of anxiety on a 4-point scale that ranges from ‘almost never’ to ‘almost always.’ This instrument was used to demonstrate that the Trier increases subjective as well as hormonal measures of stress.
Cortisol and progesterone were measured by radioimmunoassay (Diagnostic Products Corporation, Inc., Los Angeles, CA). Intra- and inter-assay coefficients of variation were <10%.
Statistical Analysis
Our primary hypotheses were that (a) high cortisol responders to the TSST would be high DA releasers in response to AMPH and (b) cortisol responses to the TSST would be positively correlated with the pleasant subjective effects of AMPH. Our secondary hypothesis was that AMPH-induced DA release would be positively associated with pleasant subjective drug effects. Primary outcome measures included DA release and TSST plasma cortisol levels. Measures of DA release for the five regions of interest (ie the anterior putamen, posterior putamen, anterior caudate nucleus, posterior caudate nucleus, and ventral striatum) and the left and right sides of each region were analyzed separately based on our prior observations of lateralization differences in findings related to regional DA release (Oswald et al, 2005). TSST plasma cortisol levels were treated as continuous variables measured over time and were also summarized as area under the curve (AUC) and peak. Trapezoidal approximation was used to calculate two AUC measures: baseline AUC (−30 to 0 min) and stress AUC (0–85 min). Similarly, two peak measures were calculated by taking the maximum attained value during the baseline and stress phases. Subjective drug responses during the PET scans were treated as continuous variables measured over time (5-point scale: 0–4) and were also summarized as AUC, calculated by trapezoidal approximation from 3 to 85 min following administration of AMPH or saline.
Summary statistics were calculated to describe the demographics of the study population. Linear regression and longitudinal regression models were used to make inferences about the primary and secondary hypotheses. All models included adjustments for age and gender. Age was included as a covariate based on considerable prior evidence that DA neurotransmission declines with age in humans (Bonci et al, 2003; Wise, 1998). Gender was included on the basis of our recent findings showing greater AMPH-induced DA release in men than women (Munro et al, 2006).
To test our first primary hypothesis, linear regression models were used to estimate the correlation between DA release (outcome) with TSST plasma cortisol levels separately for the baseline and stress phases of the TSST, adjusting for age and gender. To estimate the correlation, these models pooled information from the baseline (−30, −15, and 0 time points) and stress (25, 40, 55, 70, and 85) phase measures of plasma cortisol. Specifically, the linear regression models included an indicator for the stress phase of the TSST (relative to the baseline phase), the plasma cortisol level (time varying), the interaction of the stress phase indicator and the plasma cortisol level, age, and gender. To further evaluate these relationships, we fit similar models in which TSST plasma cortisol levels were replaced with the summary measures (AUC and peak). To test the second primary hypothesis, longitudinal regression models were used to correlate TSST cortisol levels during both the baseline and stress phases with subjective drug responses to AMPH. Summary measures from the TSST and PET were used in these analyses since measurements recorded during the TSST and PET scans were carried out on different time scales. Specifically, TSST cortisol AUC (measured during the baseline and stress phase) was modeled as an indicator for the stress phase of the TSST, the subjective drug response to AMPH (AUC), and the interaction of the indicator for the stress phase and the subjective drug response, adjusting for saline-induced subjective drug response, age, and gender. A separate model was run for each drug effect. Inferences from these models include adjustment for the correlation between the TSST cortical AUC during the baseline and stress phase. Bonferroni corrections were used to adjust findings related to the primary hypotheses for multiple comparisons.
Linear regression models were used to correlate AMPH-induced DA release with subjective drug responses (secondary hypothesis). DA release was treated as the outcome variable and the AMPH-induced and saline-induced subjective drug response was treated as both a time-varying covariate and as a summary measure (AUC and peak). In these models, the correlation between DA release and subjective drug responses was calculated after adjusting for saline-induced subjective drug response, age, and gender.
RESULTS
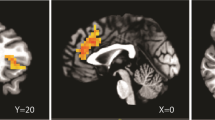
The 25 study participants were healthy Caucasians, mostly male (68%), and had a mean age of 22 years (range 18–29 years). Figure 1 shows DA D2/D3 receptor availability during the placebo and AMPH challenge and the volumes under investigation. Table 1 displays [11C]raclopride BPs as well as changes in BP values for all volumes of interest. Significant decreases were observed in [11C]raclopride BP from the saline to the AMPH scan in all volumes of interest. Figure 2 displays the mean plasma cortisol levels during the TSST. There was a significant increase in cortisol levels following the speech and mental arithmetic tasks relative to baseline levels (t=4.34, p<0.001).
Dopamine D2 receptor availability during the saline and AMPH challenge scans and the volumes under investigation (outlined). VS defining lines are displayed on an MRI coronal image, namely a bisector of the internal capsule (IC bisector) and VS separator, which is perpendicular to the IC bisector and passes through the dorsal corner of the lateral ventricles (Baumann et al, 1999).
TSST Plasma Cortisol Levels and DA Release
Table 2 displays the estimated associations between AMPH-induced changes in [11C]raclopride BP and TSST cortisol levels in several regions of the striatum. Separate analyses were performed for each of striatal region and the left and right sides of each region. Statistical significance was based on a Bonferroni corrected significance level of 0.01 (0.05 divided by five brain regions). We did not impose a Bonferroni correction across analyses for the left and right regions as preliminary findings showed that DA release values across the right and left sides of each region were highly correlated. Adjustment for each side of the region would seriously inflate the probability of type II errors.
As shown in Table 2, our findings showed statistically significant associations between TSST cortisol levels and AMPH-induced changes in [11C]raclopride BP in several regions of the striatum. Owing to its established relevance as a substrate for drug reinforcement, we were particularly interested in the findings for the ventral striatum. We found that both baseline and poststress cortisol levels were positively correlated with DA release in the left ventral striatum and the whole ventral striatum.
To corroborate findings from the linear regression analysis, the cortisol summary scores of AUC and peak were calculated. Again both baseline and poststress cortisol measures correlated with DA release in the left ventral striatum. The age and gender-adjusted associations between DA release and AUC and peak TSST cortisol are presented in Figure 3. Associations between TSST cortisol levels and DA release were not significant in the right ventral striatum. However, this lateralization of findings did not seem to generalize to the dorsal striatum (Table 2).
None of the striatal region baseline BPs (placebo scan) correlated with baseline cortisol or poststress cortisol.
TSST Plasma Cortisol Levels and Subjective Drug Responses
The mean subjective drug responses to saline and AMPH over time are displayed in Figure 4. Little effect on subjective drug responses was noted during the saline scan. As there was little variation in the ‘distrust’ response during either scan, this subjective drug response was not included in any further analyses.
Table 3 displays the correlation coefficients for the baseline and poststress TSST cortisol levels (AUC) and subjective responses (AUC) to AMPH adjusted for age, gender, and saline-induced subjective response (AUC). We set a significance level of 0.025 (0.05 divided by 2) for these analyses based on preliminary findings that showed the five positive scales were highly correlated as was generally true for the five negative scales. We found statistically significant positive correlations between the stress-induced TSST cortisol levels (AUC) and the ‘high,’ ‘good,’ ‘liking,’ and ‘rush’ subjective drug responses. These associations are displayed in Figure 5. We also found a statistically significant association between the stress-induced TSST cortisol levels and the ‘fidgety’ subjective drug response (p=0.016). There were no statistically significant associations found between baseline cortisol levels and the subjective drug responses to AMPH.
PET DA Release and Subjective Drug Responses
As previously reported (Oswald et al, 2005; Munro et al, 2006), we found statistically significant positive correlations between positive subjective drug effects and striatal DA release during the AMPH scan (significant p-values ranged from 0.029 to <0.001). This was true for all of the regions of interest. However, the positive associations were lateralized to the left side of the ventral striatum and anterior caudate.
DISCUSSION
Clinical investigation has confirmed the popular belief that stress contributes to the development, maintenance, and outcome of substance use disorders in humans (Karlsgodt et al, 2003; Sussman and Dent, 2000). Findings from human laboratory studies have further shown that stress increases drug craving (Sinha et al, 2003) and alters subjective responses to drugs of abuse (Soderpalm et al, 2003). Given the important role that the mesocorticolimbic DA system has been shown to play in substance abuse and growing evidence that stress can have profound effects on a number of neurobiological processes, it was reasonable that investigators would begin to speculate that associations between stress and substance abuse may be mediated by alterations in dopaminergic neurotransmission.
Recent human studies have utilized imaging technology to examine the influence of glucocorticoids and stress on the CNS and mesolimbic DA release. Studies have shown that glucocorticoid administration alters metabolism and blood flow in the prefrontal cortex and several other regions (de Quervain et al, 2003; Stark et al, 2006). Pruessner et al (2004) showed that ventral striatal DA release was increased in response to a psychosocial stressor in humans who reported insufficient early life maternal care. Consistent with these findings, we demonstrated that ventral striatal DA release and cortisol secretion were correlated following AMPH administration, and that both measures were associated with positive subjective responses to AMPH (Oswald et al, 2005). In the present study, we extended our earlier findings by provoking cortisol secretion through psychological mechanisms. We used the TSST to examine whether cortisol responses to psychological stress were associated with DA and/or subjective responses to AMPH. The TSST is a well-validated procedure that has been widely used to evoke the stress response in the human laboratory. Cortisol responses to this stress procedure have been demonstrated to be stable over time, even when repeated three times over a 3-month interval (Schommer et al, 2003). Although there is some cortisol habituation between sessions, high cortisol producers remain high cortisol producers and vice versa for low cortisol producers. The Trier serves as a biomarker to predict future and past cortisol responses to psychosocial stress over a significant time period.
Several important observations were derived from this study. Notably, we found that baseline and/or stress-induced cortisol levels were positively correlated with AMPH-induced DA release throughout the regions of the striatum including the ventral striatum, which contains the nucleus accumbens. Second, stress-induced cortisol levels were positively associated with the pleasant effects of AMPH. As the study design allowed for cortisol levels to be obtained for up to a month prior or after the PET procedures, the results suggest that the observed relationships between cortisol and DA release are not based solely on acute mechanisms. The fact that both baseline and stress-induced cortisol levels correlated with AMPH-induced DA release suggests that ambient cortisol concentrations over time may influence or sensitize mesolimbic dopaminergic transmission. Supporting this idea are preclinical studies showing that both stress and glucocorticoids can enhance mesolimbic DA responses (Piazza et al, 1996; Cadoni et al, 2003; Saal et al, 2003; Barrot et al, 2000; Cho and Little, 1999; Cuadra et al, 2001; Piazza and Le, 1998; Marinelli et al, 1994; Cador et al, 1993). Taken together results from our study and the preclinical findings suggest that high cortisol secretors are high DA releasers and experience greater subjective effects from psychostimulants than low cortisol secretors. This clinical finding is also supported by preclinical studies showing that stress cross-sensitizes with both psychostimulants (Kosten et al, 2003) and alcohol (Yavich and Tiihonen, 2000) leading to greater increases in striatal DA concentrations following drug administration. We did not find an association between baseline BP and cortisol measures in the present study indicating that cortisol levels may not influence striatal D2/D3 DA receptor binding availability in healthy young adults. As previously demonstrated (Munro et al, 2006; Oswald et al, 2005), we found that DA release throughout the striatum correlated with positive subjective responses to AMPH.
Although we found a positive association between stress-induced cortisol levels and AMPH-induced DA release in this study, the clinical implications of this finding remain unclear. Importantly, it has yet to be established whether high or low DA neurotransmission influences vulnerability for substance use disorders.
In this study we also found that stress-induced cortisol and positive subjective drug effects were positively correlated with left, but not with right ventral striatal DA release. We reported similar findings in our prior examination of associations between AMPH-induced cortisol responses and ventral striatal DA release (Oswald et al, 2005). The mechanism that explains this lateralization of findings is not clear. However, it should be noted that lateralization differences have been reported in glucose metabolism in the orbitofrontal cortex in humans following cocaine (Volkow et al, 2003), as well as in cerebral blood flow in the prefrontal (Tiihonen et al, 1994) and posterior (Wendt et al, 1994) cortex following alcohol. There is also evidence that specific binding of DA D2/D3 receptors is decreased in the left temporal brain (Kuikka et al, 2000) and that presynaptic DA function is diminished in the left caudate of type 1 alcoholics (Tiihonen et al, 1998).
In addition to the correlation of cortisol and DA in the ventral striatum, we also found a correlation between cortisol and DA release in the caudate and putamen nuclei. The ventral striatum houses the nucleus accumbens and therefore the significance of the findings may relate to behaviors associated with reward and reinforcement as discussed above. In contrast, it is possible that the correlation of cortisol and DA in the caudate and putamen might best relate to cognitive functioning, and in particular those cognitive skills that are most often subsumed under the term ‘executive’ functioning. The role of striatal DA to cognitive function has recently become a focus of functional neuron-imaging studies (see Cropley et al, 2006, for a review). These studies have documented the association between cognition and measures of presynaptic as well as postsynaptic striatal DA. In general, reduced DA uptake, decreased DA binding, and decreased tonic DA release in the caudate and putamen are associated with worse performance on tasks requiring set shifting, planning, working memory, and sustained attention (ie executive skills) in healthy adults (Erixon-Lindroth et al, 2005; Mehta et al, 2004; Mozley et al, 2001; Verhoeff et al, 2001; Volkow et al, 1998). These findings are corroborated by research examining the effects of structural lesions in the caudate and putamen on executive function (Monchi et al, 2006; Mesulam, 2000; Iversen, 1979). Taken together, studies investigating the consequences of structural damage to the caudate and putamen and functional neuroimaging studies of the role of striatal DA converge on the conclusion that the striatum, and specifically, striatal dopaminergic function, appears important to cognition, particularly on executive tasks. How the degree of cortisol exposure may affect DA dynamics and therefore certain aspects of cognitive function is an area that needs exploration.
A limitation of the present study is that firm conclusions about the causal nature of the relationship between glucocorticoids and drug reinforcement cannot be established owing to the correlational nature of the design. There is considerable evidence that both extrahypothalamic and hypothalamic CRH mediate the actions of drugs of abuse (Sarnyai et al, 2001). Thus, activation of CRH pathways may be the primary mediator of stress-induced sensitization to drugs and glucocorticoids merely the surrogates of this relationship. Another caveat of the present study is that the placebo scan was always conducted before the AMPH scan because a single dose of AMPH can sensitize DA transmission for a prolonged time period. Thus, the fixed order of scans eliminated potential carryover effects of AMPH. Although attempts were made to establish a ‘quiet’ cortisol baseline for the Trier by having subjects first undergo the ‘passive’ Trier as described in methods, it is possible that baseline cortisol levels still reflect anticipatory anxiety associated with the procedure. Finally, our sample was composed of healthy subjects. A similar study should be completed with a group of psychostimulant users to determine if DA measurements predict consumption and/or relapse (Sinha et al, 2006).
In summary, the findings show that cortisol levels in response to the psychological stress test were positively associated with AMPH-induced DA release in the striatum. Subjects with greater cortisol responses to the stress test also reported more positive subjective drug effects than subjects with lower cortisol responses. Higher ratings of positive drug effects were positively associated with greater DA release throughout the striatum. Our findings provide evidence of interrelationships among glucocorticoid levels, subjective responses to AMPH, and brain DA release in humans.
References
Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV (2000). The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci 12: 973–979.
Baumann B, Danos P, Krell D, Diekmann S, Leschinger A, Stauch R et al (1999). Reduced volume of limbic system-affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. J Neuropsychiatry 11: 71–78.
Bonci A, Bernardi G, Grillner P, Mercuri NB (2003). The dopamine-containing neuron: maestro or simple musician in the orchestra of addiction? Trends Pharmacol Sci 24: 172–177.
Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF et al (2005). Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res 29: 185–195.
Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger Jr JI et al (1994). A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol 55: 149–158.
Cadoni C, Solinas M, Valentini V, Di CG (2003). Selective psychostimulant sensitization by food restriction: differential changes in accumbens shell and core dopamine. Eur J Neurosci 18: 2326–2334.
Cador M, Cole BJ, Koob GF, Stinus L, Le MM (1993). Central administration of corticotropin releasing factor induces long-term sensitization to D-amphetamine. Brain Res 606: 181–186.
Cho K, Little HJ (1999). Effects of corticosterone on excitatory amino acid responses in dopamine-sensitive neurons in the ventral tegmental area. Neuroscience 88: 837–845.
Cropley VL, Fujita M, Innis RB, Nathan PJ (2006). Molecular imaging of the dopaminergic system and its association with human cognitive function. Biol Psychiatry 59: 898–907.
Cuadra G, Zurita A, Gioino G, Molina V (2001). Influence of different antidepressant drugs on the effect of chronic variable stress on restraint-induced dopamine release in frontal cortex. Neuropsychopharmacology 25: 384–394.
de Quervain DJ, Henke K, Aerni A, Treyer V, McGaugh JL, Berthold T et al (2003). Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur J Neurosci 17: 1296–1302.
Deroche-Gamonet V, Sillaber I, Aouizerate B, Izawa R, Jaber M, Ghozland S et al (2003). The glucocorticoid receptor as a potential target to reduce cocaine abuse. J Neurosci 23: 4785–4790.
Endres CJ, Kolachana BS, Saunders RC, Su T, Weinberger D, Breier A et al (1997). Kinetic modeling of [11C]raclopride: combined PET-microdialysis studies. J Cereb Blood Flow Metab 17: 932–942.
Erixon-Lindroth N, Farde L, Wahlin TB, Sovago J, Halldin C, Backman L (2005). The role of the striatal dopamine transporter in cognitive aging. Psychiatry Res 138: 1–12.
Federenko IS, Nagamine M, Hellhammer DH, Wadhwa PD, Wust S (2004). The heritability of hypothalamus pituitary adrenal axis responses to psychosocial stress is context dependent. J Clin Endocrinol 89: 6244–6250.
Goeders NE, Peltier RL, Guerin GF (1998). Ketoconazole reduces low dose cocaine self-administration in rats. Drug Alcohol Depend 53: 67–77.
Gordon W (2002). Early environmental stress and biological vulnerability to drug abuse. Psychoneuroendocr 27: 115–126.
Iversen L (1979). The chemistry of the brain. Sci Am 241: 134–149.
Karlsgodt KH, Lukas SE, Elman I (2003). Psychosocial stress and the duration of cocaine use in non-treatment seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse 29: 539–551.
Kirschbaum C, Pirke KM, Hellhammer DH (1993). The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28: 76–81.
Kosten TA, Zhang XY, Kehoe P (2003). Chronic neonatal isolation stress enhances cocaine-induced increases in ventral striatal dopamine levels in rat pups. Brain Res Dev Brain Res 141: 109–116.
Kuikka JT, Repo E, Bergstrom KA, Tupala E, Tiihonen J (2000). Specific binding and laterality of human extrastriatal dopamine D2/D3 receptors in late onset type 1 alcoholic patients. Neurosci Lett 292: 57–59.
Kuwabara H, Rousset OKA, Alexander M, Wong DF (2004). A method for estimating accuracy of intra and inter modality image volume registration. J Nucl Med 45: 393.
Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A (2002). Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology 27: 1027–1035.
Marinelli M, Piazza PV, Deroche V, Maccari S, Le MM, Simon H (1994). Corticosterone circadian secretion differentially facilitates dopamine-mediated psychomotor effect of cocaine and morphine. J Neurosci 14: 2724–2731.
Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y et al (2004). Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology 29: 1190–1202.
Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y et al (2003). Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 23: 285–300.
Mehta MA, Manes FF, Magnolfi G, Sahakian BJ, Robbins TW (2004). Impaired set-shifting and dissociable effects on tests of spatial working memory following the dopamine D2 receptor antagonist sulpiride in human volunteers. Psychopharmacology (Berl) 176: 331–342.
Mesulam M (2000). Brain, mind, and the evolution of connectivity. Brain Cogn 42: 4–6.
Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J (2006). Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol 59: 257–264.
Mozley LH, Gur RC, Mozley PD, Gur RE (2001). Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry 158: 1492–1499.
Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J et al (2006). Sex differences in striatal dopamine release inhealthy adults. Biol Psychi 59: 966–974.
Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L et al (2005). Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology 30: 821–832.
Piazza PV, Le MM (1998). The role of stress in drug self-administration. Trends Pharmacol Sci 19: 67–74.
Piazza PV, Marinelli M, Rouge-Pont F, Deroche V, Maccari S, Simon H et al (1996). Stress, glucocorticoids, and mesencephalic dopaminergic neurons: a pathophysiological chain determining vulnerability to psychostimulant abuse. NIDA Res Monogr 163: 277–299.
Pruessner JC, Champagne F, Meaney MJ, Dagher A (2004). Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci 24: 2825–2831.
Saal D, Dong Y, Bonci A, Malenka RC (2003). Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37: 577–582.
Sarnyai Z, Shaham Y, Heinrichs SC (2001). The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev 53: 209–243.
Schad CA, Justice Jr JB, Holtzman SG (2002). Endogenous opioids in dopaminergic cell body regions modulate amphetamine-induced increases in extracellular dopamine levels in the terminal regions. J Pharmacol Exp Ther 300: 932–938.
Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D (2001). Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J Neurosci 21: 5916–5924.
Schommer NC, Hellhammer DH, Kirschbaum C (2003). Dissociation between reactivity of the hypothalamus–pituitary–adrenal axis and the sympathetic–adrenal–medullary system to repeated psychosocial stress. Psychosom Med 65: 450–460.
Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ (2006). Stress-induced cocaine craving and hypothalamic–pituitary–adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 63: 324–331.
Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ (2003). Hypothalamic–pituitary–adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology 170: 62–72.
Soderpalm A, Nikolayev L, de WH (2003). Effects of stress on responses to methamphetamine in humans. Psychopharmacology 170: 188–199.
Spielberger CD (1983). State-Trait Anxiety Inventory (Form Y). Consulting Psychologists Press: Palo Alto, CA.
Stark R, Wolf OT, Tabbert K, Kagerer S, Zimmermann M, Kirsch P et al (2006). Influence of the stress hormone cortisol on fear conditioning in humans: evidence for sex differences in the response of the prefrontal cortex. Neuroimage 32: 1290–1298.
Sussman S, Dent CW (2000). One-year prospective prediction of drug use from stress-related variables. Subst Use Misuse 35: 717–735.
Tiihonen J, Kuikka J, Hakola P, Paanila J, Airaksinen J, Eronen M et al (1994). Acute ethanol-induced changes in cerebral blood flow. Am J Psychiatry 151: 1505–1508.
Tiihonen J, Vilkman H, Rasanen P, Ryynanen OP, Hakko H, Bergman J et al (1998). Striatal presynaptic dopamine function in type 1 alcoholics measured with positron emission tomography. Mol Psychiatry 3: 156–161.
Verhoeff NP, Kapur S, Hussey D, Lee M, Christensen B, Papatheodorou G et al (2001). A simple method to measure baseline occupancy of neostriatal dopamine D2 receptors by dopamine in vivo in healthy subjects. Neuropsychopharmacology 25: 213–223.
Volkow ND, Fowler JS, Wang GJ, Swanson JM (2004). Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry 9: 557–569.
Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS et al (1998). Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 155: 344–349.
Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L et al (2003). Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci 23: 11461–11468.
Wendt PE, Risberg J, Stenberg G, Rosen I, Ingvar DH (1994). Ethanol reduces asymmetry of visual rCBF responses. J Cereb Blood Flow Metab 14: 963–973.
Wise RA (1998). Drug-activation of brain reward pathways. Drug Alcohol Depend 51: 13–22.
Yavich L, Tiihonen J (2000). Ethanol modulates evoked dopamine release in mouse nucleus accumbens: dependence on social stress and dose. Eur J Pharmacol 401: 365–373.
Acknowledgements
This work was supported by AA10158 (GSW), AA12837 (MEM), AA12839 (DFW) and GCRC (NIH/NCRR M01RR00052).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wand, G., Oswald, L., McCaul, M. et al. Association of Amphetamine-Induced Striatal Dopamine Release and Cortisol Responses to Psychological Stress. Neuropsychopharmacol 32, 2310–2320 (2007). https://doi.org/10.1038/sj.npp.1301373
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301373
Keywords
This article is cited by
-
Cannabidiol versus placebo as adjunctive treatment in early psychosis: study protocol for randomized controlled trial
Trials (2023)
-
Modulation of behavioral and neurochemical responses of adult zebrafish by fluoxetine, eicosapentaenoic acid and lipopolysaccharide in the prolonged chronic unpredictable stress model
Scientific Reports (2021)
-
High Recreational Gamblers Show Increased Stimulatory Effects of an Acute Laboratory Gambling Challenge
Journal of Gambling Studies (2021)
-
Early Life Stress and Substance Use Disorders: Underlying Neurobiology and Pathways to Adverse Outcomes
Adversity and Resilience Science (2020)
-
The relationship between childhood trauma, dopamine release and dexamphetamine-induced positive psychotic symptoms: a [11C]-(+)-PHNO PET study
Translational Psychiatry (2019)