Abstract
Schizophrenia, many believe, reflects an enhanced vulnerability to psychological stress. Controlled exposure to stressors, however, has produced inconclusive results, particularly with regards to neurohormones. Some of the variability may be attributable to the nature and psychological significance of the stimulus and failure to control physiologic confounds. In addition, it is possible that the heterogeneity of schizophrenia is an important factor. In a carefully designed study and in a controlled setting, we measured the neuroendocrine response of eight polydipsic hyponatremic (PHS), seven polydipsic normonatremic (PNS), and nine nonpolydipsic normonatremic (NNS) (ie normal water balance) schizophrenic in-patients as well as 12 healthy controls (HC) to two different stressors: one of which appears to influence neuroendocrine secretion through its psychological (cold pressor) and the other (upright posture) through its systemic actions. Subjects in the three psychiatric groups were stabilized and acclimated to the research setting, and all received saline to normalize plasma osmolality. Following the cold pressor, plasma adrenocorticotropin and cortisol levels showed a more prolonged rise in PHS patients relative to PNS patients. NNS patients, in contrast, exhibited blunted responses relative to both of the polydipsic groups and the HC. Peak vasopressin responses were also greater in PHS and blunted in NNS patients. Responses to the postural stimulus were similar across patient groups. These findings provide a mechanism for life threatening water intoxication in schizophrenia; help to reconcile conflicting findings of stress responsiveness in schizophrenia; and potentially identify a discrete patient subset with enhanced vulnerability to psychological stress.
Similar content being viewed by others
INTRODUCTION
The hypothesis that schizophrenia is characterized by an enhanced vulnerability to psychological stress has evolved over time but consistently has been attributed to hippocampal dysfunction (Mednick and Schulsinger, 1968; Gray, 1998; Walker and Diforio, 1997; Moghaddam, 2002; Myin-Germeys et al, 2005). Changes in hypothalamic–pituitary–adrenal (HPA) axis and arginine vasopressin (AVP: antidiuretic hormone) activity, stress sensitive markers of hippocampal function (Herman et al, 2002, 2005; Nettles et al, 2000; Onaka and Yagi, 1998), however, generally are normal or even blunted in schizophrenia (Albus et al, 1982; Breier et al, 1988; Gispen-de Wied, 2000; Jansen et al, 1998, 2000; Kudoh et al, 1999; Marcelis et al, 2004). Previous reports of enhanced activity may instead be attributable to responses to specific stressors, systemic effects of the stimulus, severity, or lability of psychiatric symptoms, or to acute hospital admission, per se (Herman et al, 2005; Walder et al, 2000; Walsh et al, 2005).
The absence of clear evidence of enhanced neuroendocrine responses in schizophrenia also leaves open the possibility that only a subset of patients exhibit enhanced vulnerability. Recognition of this possibility has led others to seek evidence of HPA axis dysfunction in patients differentially exposed to factors thought to influence hippocampal function (Braehler et al, 2005). In this regard, hippocampal function varies along its longitudinal axis in both rodents (Bannerman et al, 2004; Moser and Moser, 1998; Risold and Swanson, 1996) and primates (Barbas and Blatt, 1995; Strange et al, 1999; Strange and Dolan, 2001), and structural findings are often more prominent in one or the other end of the hippocampus in patients (Narr et al, 2004; Pegues et al, 2003; Szeszko et al, 2003; Velakoulis et al, 2001; Weiss et al, 2005). The ventral segment in rodents (analogous to anterior segment in primates (Rubin et al, 1966)) modifies neuroendocrine (Herman et al, 1995, 2005; Mueller et al, 2004; Nettles et al, 2000) and behavioral (Flores et al, 2005; Trivedi and Coover, 2004) responses to psychological but not physical stresses (but see Tuvnes et al, 2003). Indeed, an animal model of schizophrenia, in which the development of this segment is disrupted in the neonatal rat (Lipska, 2004), exhibits increased AVP and HPA axis responses to a psychological stimulus (Chrapusta et al, 2003; Mitchell and Goldman, 2004) as well as enhanced behavioral (Flores et al, 2005) responses to psychological stress. Thus one might predict that only patients with prominent anterior hippocampal pathology would exhibit increased vulnerability to psychological stress.
Diminished volume of the anterior hippocampus is particularly prominent in schizophrenic patients with polydipsia and hyponatremia (Elkashef et al, 1996; Luchins et al, 1997; Goldman et al, 2005). About 20% of schizophrenic patients drink excessive amounts of water (‘primary polydipsia’) and about 20% of these retain enough of this excess water to produce a dilutional hyponatremia (de Leon et al, 1994). Furthermore, polydipsic patients with and without hyponatremia display impaired HPA axis negative feedback (Goldman et al, 1993) that is consistent with hippocampal pathology (Goldman et al, 2006). In addition, hyponatremic polydipsic (but not normonatremic polydipsic) patients exhibit an unexplained increase in AVP secretion that contributes to their water retention (Goldman et al, 1988, 1996). Finally, this defect worsens with the stress of acute psychosis in the hyponatremic subset, culminating in episodes of life-threatening water intoxication (de Leon et al, 1994; Goldman et al, 1997).
Based on the (1) previous reports of blunted neuroendocrine responses to psychological stress in schizophrenia; (2) evidence that ventral/anterior hippocampal dysfunction enhances neuroendocrine responses to psychological stress; and (3) evidence that patients with water imbalance exhibit signs of anterior hippocampal pathology and enhanced neuroendocrine activity, summarized above, we tested the following hypotheses: AVP and HPA axis responses to psychological stress will be enhanced in (1) polydipsic hyponatremic (PHS) schizophrenic relative to polydipsic normonatremic (PNS) schizophrenic patients, (2) these two groups relative to healthy controls (HC), and (3) these three groups relative to nonpolydipsic normonatremic (NNS) schizophrenic patients (ie a sample representative of the majority of patients who have normal water balance). In addition, we predicted that the neuroendocrine responses to a systemic stimulus would not differ in these groups.
As psychological stress responses are heavily influenced by perceptions of reality, and reality testing is, by definition, distorted in schizophrenia, standardizing a psychological stimulus for this population is a daunting challenge. In fact, schizophrenic patients are often quite adept at simply ignoring psychological stimuli (Dawson and Nuechterlein, 1984; Jansen et al, 2000; Zahn and Pickar, 2005). Psychological stressors are stimuli that signal, but do not in themselves constitute, a potential threat to homeostasis. The recognized attributes of a psychological stressor include aversiveness (Peters et al, 1998), and neuroendocrine components that both anticipate (Gaab et al, 2005) as well as habituate (Herman et al, 2005) to the stimulus. We chose the cold pressor (ie immersion of a limb in ice water) because it exhibits these attributes (al'Absi and Petersen, 2003; Bullinger et al, 1984; Ehrenreich et al, 1997; Farhadi et al, 2005; Gregg et al, 1999; Lovallo, 1975) yet is difficult to ignore. In fact, the response is independent of the subjects' attentiveness (Hodes et al, 1990) and is predicted by the duration of exposure to the stimulus (Lovallo, 1975). Equally important is that data suggest that the systemic actions of the cold pressor (ie cold, elevated blood pressure) have no effect or may actually blunt HPA secretion (Edelson and Robertson, 1986; Kendler et al, 1978; Wittert et al, 1992). For the systemic stimulus, we chose becoming upright after a period of recumbency (postural stimulus) because it lacks these attributes of a psychological stimulus (Hennig et al, 2000), and the neuroendocrine response is accounted for by its systemic effects (Hennig et al, 2000; Jacob et al, 1998).
PATIENTS AND METHODS
Subjects
Psychiatric subjects were recruited from psychiatric in-patient and outpatient facilities. Subjects were grouped by a two-step procedure before and following transfer to the Psychiatric Clinical Research Center at University of Illinois at Chicago (UIC). All had primary diagnoses of schizophrenia or schizoaffective disorder and were without major medical or neurological disorders, and were not taking corticosteroids or medications with recognized effects on AVP function (Goldman et al, 1997). Potential subjects with polydipsia and hyponatremia had unexplained plasma sodium levels ⩽125 mEq/l, and multiple urine specific gravities <1.008 (hyposthenuria); those with polydipsia and normonatremia had no plasma sodium levels ⩽135 mEq/l, but a similar history of hyposthenuria; those with normal water balance had no sodium levels ⩽135 mEq/l or urine-specific gravity levels <1.015. Informed witnessed written consent was obtained after the Institutional Review Boards of the University of Chicago (UC) and UIC approved the studies. Informed consent was assessed by determining the ability to repeat key elements of the consent form, performance on cognitive screening tasks, involvement of family and other patient advocates, and regular reassessment of willingness to continue with the research.
During the first 3 weeks of admission, subjects were acclimated to the research setting and psychotropic medication doses were optimized. Haloperidol was the preferred antipsychotic, but patients could receive olanzapine or risperidone if they had had adverse reactions to first generation agents. Mood instability was treated with valproic acid (see legend Table 1). Final patient assignment into PHS, PNS, or NNS schizophrenic groups was based on spot urine and plasma samples obtained three times weekly during the first 3 weeks of hospitalization. PHS had mean urine osmolalities mmol/kg (Uosm) <300 mmol/kg and mean plasma osmolalities (Posm) <285 mmol/kg and one or more Posms <275 mmol/kg; PNS met the same criteria for mean Uosm, but their mean Posm >285; NNS had mean Uosm >500 mmol/kg and mean Posm >285 mmol/kg. Measures of urine dilution have been shown to reliably predict fluid intake (Shutty et al, 1997). Thus all PHS patients had a history of moderately severe hyponatremia and polydipsia, but at the time of the study may only have been episodically hyponatremic/hypoosmolemic and moderately polydipsic (Shutty et al, 1997).
During the fifth week of admission, trained raters administered the Positive and Negative Symptom Scale (PANSS) (Kay et al, 1987), Hamilton Depression Scale (HAM-D) (Bech et al, 1986), and the Global Assessment of Functioning Scale (GAF) (Endicott et al, 1976). Patients underwent an MRI scan during the fourth week as part of a related study (Goldman et al, 2005). Psychiatric diagnosis was confirmed at discharge in a multidisciplinary conference and relied largely on the Structured Clinical Interview for DSM-IV (Spitzer et al, 1992).
HC were recruited by advertisements placed around the University communities as described previously (Goldman et al, 1996) and were studied as outpatients. Each was administered the SCID-NP by a trained rater (Version 2.0) (First et al, 1996), and none had current or past axis I disorders, or medical conditions or were taking medication known to alter AVP function. We attempted to match groups on age and gender.
Procedures
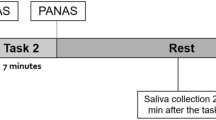
Studies were performed in the evening because that is when HPA axis activity is most stable and hippocampal influences are thought to be most apparent (van Eekelen et al, 2003). In order to further stabilize basal HPA axis activity, subjects were acclimated to the Clinical Research Center before the study. On the day of the study, subjects refrained from smoking and eating after 1200 hours. After a light low-carbohydrate dinner at 1700 hours, subjects assumed a supine posture and two intravenous catheters were placed after which a baseline Posm was determined (Figure 1). Starting at 1800 hours, 0.45%, 0.9%, or 3.0% saline were infused at a rate calculated to produce a Posm of between 285 and 290 mmol/kg by 1900 hours. By normalizing Posm, we controlled for the major factor that has been demonstrated to modulate AVP responses to non-osmotic stimuli in normals (Robertson, 2001) as well as in those with schizophrenia (Goldman et al, 1996, 1997).
Time line of psychological (cold pressor) and systemic (postural) stressors. Study was conducted in the evening when HPAA axis is most stable. Subjects assumed a supine posture before placement of intravenous catheters at 1730 hours. At 1800 hours subjects received a 1-h infusion of saline to normalize Posm. One hour later they submerged a hand in an ice water bath for 60 s. Blood samples and assessments were generally obtained at 15 min intervals from 30 min before until 105 min following the cold pressor. Four and one-half hours after becoming supine, subjects stood up, and samples were obtained at 5 min intervals for the next 20 min.
Sampling and assessments began at 1930 hours and continued until 2220 hours. At each sampling time, blood was drawn, and vital signs (Dinamap XL Vital Signs Monitor; Critikon, Tampa, FL), and presence of nausea or light-headedness were recorded. At 2000 hours subjects placed their non-dominant hand in ice water up to the wrist, and were encouraged to keep it immersed for 1 min. Immediately afterward, they were asked how painful the stimulus felt on a scale of 1–4. For the postural stimulus, subjects stood up for 20 min after the 2200 hours sample was taken (Figure 1). Sampling and assessments generally occurred at 15-min intervals before and following the cold pressor, except that the last blood sample before the stimulus occurred at −10 min, and additional blood samples were obtained 5 and 10 min following the stimulus. For the postural stimulus, a baseline sample was obtained, plus three additional samples at 5-min intervals starting 10 min after subjects stood up (Figure 1).
Psychiatric subjects were studied during the fifth week of their admission after being acclimated to the research setting and stabilized on medication. All received the cold pressor. HC were studied on two outpatient visits. In randomly assigned order, they immersed their hands in the ice bath on one visit and in tepid water on the other. This enabled us to both characterize the normal response to the cold stressor and to refine the number of time points to include for each repeated measure analysis.
Laboratory
Collected blood samples were processed as described previously (Goldman et al, 1988). Posm, cortisol, and vasopressin were measured in the Clinical Research Center core labs at UIC (freezing point depression, Advanced Instruments 3D3, Needham, MA); UC (Chemiluminescent automated immunoassay, DPC, Immulite 1000, Los Angeles, CA); and Northwestern University (RIA) (Goldman et al, 1996), respectively. Plasma adrenocorticotropin (ACTH) was measured at UC endocrine laboratory (Chemiluminescent automated immunoassay, Nichols Advantage, San Clemente, CA).
Data Analysis
We took the natural log of cortisol and vasopressin levels to normalize the data. No other measures were transformed. A mixed effect linear regression model was chosen to analyze responses for each repeatedly sampled measure (Gibbons et al, 1993; Hedeker and Gibbons, 1996a). By modeling the shape of each subject's response, rather than measuring the deviation from the group norm, the accuracy and power of the analysis is greater than that of analysis of variance for repeated measures (Hedeker and Gibbons, 2006). In so doing, this approach also handles missing data, intra-subject correlation, and time-varying covariates in a more flexible and realistic manner.
For the cold pressor, the number of time points for each measure includes all values from −30 min until and including the time point where the group's responses to the cold pressor and control condition appeared to be identical (see Supplementary Figure 1). For the postural stimulus, all timed samples were included in the analyses. To characterize the difference in the pattern of each group's response, the shape of the response was decomposed into linear, quadratic, and cubic components. These three time trends and the intercept were entered as random effects in the model. Only the significance of the linear and quadratic trends and their interactions (reflecting differences in the slope and peak of the responses, respectively), however, were considered of interest. Power was maximized by limiting the statistical assessment to three a priori group contrasts in order to test if: (1) the PHS group's responses exceeded those of the PNS group; (2) these two polydipsic groups' responses exceeded those of the HC; and (3) these three groups responses exceed those of the NNS patients.
These three group (Difference) contrasts and their nine interactions with the time trends were entered as fixed effects. Significant main effects are only reported below when interaction terms were insignificant. To make the Results section easier to follow, we translate group by time trend interactions in the text (‘higher peak response in the two polydipsic groups compared to the HC’), rather than just listing the specific contrast (eg ‘quadratic time trend interaction with group contrast, which compared the two polydipsic groups to HC’). The two conditions (cold pressor, tepid water) in HC were analyzed in an analogous manner to that described above for the group analysis, but the actual contrasts and interactions are listed in the Results section as they are easier to grasp. All effects are reported as Z-scores. Covariates were examined by adding them as additional fixed effects to the model and assessing the significance of their contribution (also are expressed as Z-scores). Non-repeated measure analyses were conducted by analysis of variance or ordinal regression (Hedeker and Gibbons, 1996b) as indicated. Probabilities reflect two-tailed distributions even though we had directional hypotheses.
RESULTS
Cold Pressor and Tepid Water Conditions: HC
HC were studied on two occasions in order to both characterize the normal response to the cold stressor, as well as to refine the number of time points to include in the repeated measures analyses. In randomly assigned order, controls immersed a hand in the ice bath on one visit and in tepid water on the other. Blood pressure increased following immersion in the ice bath (−10 to +1 min: 9.1±13.6 mmHg) but did not change in the tepid water condition (−1.7±5.0 mmHg; time by condition interaction: Z=2.35, p=0.019). Plasma ACTH rose before, as well as transiently following, the cold pressor but did not appear to change in the control (tepid water) condition (quadratic trend by condition interaction: Z=2.36, p=0.018, Supplementary Figure 1a). Plasma cortisol appeared to lag ACTH following the cold pressor before slowly returning to baseline, and also did not appear to change in the tepid water condition (Supplementary Figure 1b, quadratic trend by condition: Z=3.27, p=0.001). Plasma vasopressin (AVP) resembled the ACTH response, that is AVP rose before and transiently following the cold pressor, and did not appear to change in the tepid water condition (Supplementary Figure 1c, quadratic trend by condition: Z=1.96, p=0.048). Heart rate, Posm, expired air carbon monoxide, glucose, plasma sodium, and blood urea nitrogen did not change and were similar in both conditions (data not shown).
Cold Pressor: Three Patient Groups and HC
The patient population consisted mostly of middle-aged subjects with chronic and disabling mental illness (Table 1). The three patient groups resembled each other on demographic and clinical measures, though PHS patients appeared to be older (t=1.92, df=9.7, p=0.084). Paranoia related items on the PANSS, a putative predictor of stress reactivity (Lopes-Machado et al, 2002), did not differ between patient groups (data not shown). Treatment with atypical vs typical antipsychotic medications did not differ between groups. Water imbalance appeared to be somewhat less severe in the two polydipsic groups than in previous studies (Goldman et al, 1996) (Table 1). Only one of the control subjects, but many of the patients, smoked tobacco products (Table 1).
Acute Response to Cold Pressor
Before the cold pressor, measures known to modulate neuroendocrine responses were similar across the four groups (Table 2). Although Posm was lower at the start of the study in the PHS subjects, this was corrected by the saline infusion (Table 2). Subjective pain responses to the cold pressor were similar across all four groups. Blood pressure responses on the other hand were significantly blunted in the NNS group (ie normal water balance) relative to the other three groups (t=2.66, df=21.3, p=0.014), who resembled each other on this measure. The two polydipsic groups kept their hands immersed in the ice bath for less time than the HC (t=2.75, df=14.5, p=0.015), whereas immersion time was intermediate in the NNS group.
HPA Axis Responses to Cold Pressor
Basal ACTH levels (−30 min) were similar in the two polydipsic groups but higher than in HC (Figure 2a, t=3.27, df=19.0, p=0.001), whereas levels were intermediate in the NNS group. PHS patients exhibited a more sustained rise in ACTH than PNS patients (Z=2.63, p=0.008) (ie hypothesis 1). The response appeared to be greater in these two groups than in the HC, but the difference did not achieve significance (p>0.05) (hypothesis 2). Both the slope (Z=2.22, p=0.026) and peak (Z=2.26, p=0.021) of the response were blunted in NNS patients relative to the other three groups (hypothesis 3). Figure 3a shows the same data as percent of the subjects' −30 min ACTH levels in order to facilitate visual comparison of the groups' response patterns. As reported previously (Lovallo, 1975), ACTH responses were predicted by the duration of hand immersion in the ice bath across subjects (covariate Z=2.30, p=0.02). Neither basal ACTH levels (−30 min), blood pressure, age, pre-infusion, or concurrent Posm, subjective pain, nor behavioral ratings were related to the ACTH responses.
Effects of cold pressor on plasma hormones and related measures. Each symbol shows the mean (±SEM) for all subjects in a given group for each variable at a different time interval before and following the cold pressor. Groups consisted of schizophrenic patients who were polydipsic and hyponatremic (PHS) ( ), polydipsic and normonatremic (PNS) (
), polydipsic and normonatremic (PNS) ( ), and nonpolydipsic and normonatremic (NNS) (□), and HC (•). Mixed effects linear regression demonstrated that hormonal responses were enhanced in PHS relative to PNS, and enhanced in both these groups and HC relative to NNS.
), and nonpolydipsic and normonatremic (NNS) (□), and HC (•). Mixed effects linear regression demonstrated that hormonal responses were enhanced in PHS relative to PNS, and enhanced in both these groups and HC relative to NNS.
Percent increase in plasma ACTH and vasopressin. Each symbol shows the percent changes from baseline. See legend of Figure 2 for explanation of symbols.
Cortisol responses were strongly predicted by ACTH levels 15 min earlier (Z=6.02, p<<0.001) but group differences did not appear to closely resemble those seen with ACTH. Thus peak levels were higher in PNS than PHS subjects, and HC responses resembled those of the two polydipsic groups. Statistical analysis, however, not only demonstrated that the peak response was lower in normonatremic patients compared to the other three groups (hypothesis 3: Z=3.69, p=0.001) (Figure 2b), but also that the rise was more sustained (ie greater slope) in the PHS than the PNS subjects (hypothesis 1: Z=3.49, p=0.001).
AVP Response to Cold Pressor
Basal AVP levels (−30 min) were diminished in the two polydipsic groups relative to HC (Supplementary Table 1, t=2.83, df=13.6, p=0.014), and were intermediate in the NNS group to the two polydipsic groups and the controls. An anticipatory (ie prestimulus) linear rise in AVP was significant (Z=3.13, p=0.001) and statistically equivalent across groups. Peak response to the cold pressor was greater in PHS than PNS patients (hypothesis 1: Z=2.04, p=0.040), and again blunted (albeit marginally) in patients with normal water balance relative to the other three groups (hypothesis 3: Figure 2c, Z=1.77, p=0.083). The responses of the HC and the two polydipsic groups were not statistically different. Group differences can again be more easily appreciated by plotting AVP as percent of the subjects' AVP level at −30 min (Figure 3b). The AVP response was predicted by basal AVP levels (covariate Z=2.01, p=0.044), pre-infusion Posm (covariate Z=2.00, p=0.044), as well as concurrent ACTH levels (Z=3.83, p=0.001). Posm rose consistently in the two polydipsic groups following the cold pressor relative to HC (Figure 2d, Z=2.61, p=0.009), who resembled the NNS patients in this regard. The change, however, did not correlate with the AVP response. Age, subjective pain, duration of immersion in the ice bath, blood pressure, and behavioral ratings were also not related to the AVP responses.
Response to Postural Stimulus
Four and one-half hours after assuming a supine posture, and 120 min after the cold pressor stimulus (Figure 1), subjects stood up and remained upright for the last 20 min of the study. One HC became frankly orthostatic and her data were excluded. No other subject reported nausea or lightheadedness at any time. Basal cardiovascular measures were similar across groups. Blood pressure remained stable upon arising and did not differ across groups (Supplementary Figure 2a). In contrast, heart rate increased more in those with polydipsia and hyponatremia than polydipsic patients without hyponatremia (Supplementary Figure 2b, Z=2.48, p=0.013), who in turn appeared to resemble the other two groups. The HC exhibited only a transient rise in ACTH, in contrast to the sustained increase seen in the two polydipsic groups (Supplementary Figure 2c, Z=2.13, p=0.03), as well as the NNS patients. In contrast, cortisol (Supplementary Figure 2d; Z=3.18, p=0.001) and AVP (Supplementary Figure 2e, Z=2.74, p=0.006) showed sustained and similar increases in all groups.
DISCUSSION
Divergent Neuroendocrine Responses to the Cold Pressor in Schizophrenia
Neuroendocrine responses to a cold pressor stimulus are enhanced in schizophrenic patients with polydipsia and hyponatremia relative to those who are normonatremic, but are blunted in patients with normal water balance. Specifically, ACTH and AVP responses are relatively prolonged and show a greater rise, respectively, in hyponatremic compared to normonatremic polydipsic patients, whereas these same measures are blunted in NNS patients relative to both polydipsic patient groups as well as to healthy comparison subjects (Figures 2a, c, 3a and b). Although more group overlap was apparent with the plasma cortisol response (Figure 2b), group differences were analogous to those seen with ACTH and indeed were highly predicted by the ACTH response. The blunted neuroendocrine response to a psychological stimulus in the patients with normal water balance along with their preserved response to a systemic stimulus reproduces previous findings in persons with schizophrenia (Jansen et al, 2000) (but see Elman et al, 1998), but the findings in the PHS patients are completely novel. In contrast our predicted differences between the two polydipsic groups and HC were not apparent. Thus, although our first and third hypotheses were supported, our second hypothesis was not.
As the subject groups were matched and the procedures were conducted in a carefully monitored setting, we can confidently address the potential confounding role of recognized factors in these findings. Olanzapine and other atypical neuroleptics have recently been shown to inhibit basal cortisol secretion in normals and those with schizophrenia (Mann et al, 2006), however, exposure to atypicals did not differ across groups (Table 1), and if anything appeared more common in those with polydipsia. Other agents appear to have minimal or no effects on neuroendocrine function in schizophrenia (Goldman et al, 1996; Kaneda et al, 2002; Kawai et al, 2002; Goldman and Hussain, 2004) and exposure to these agents, in any case, was also similar across patient groups (Table 1). Likewise, psychiatric symptoms did not differ across patient groups, though we cannot exclude the possibility that undetected symptoms (eg repetitive behaviors) may have contributed (Luchins, 1990). Although hyponatremic subjects appeared to be older (Table 1), including age as a covariate did not influence the findings (Otte et al, 2005; Stout et al, 1999) nor would any effect be expected from this small an age difference (Otte et al, 2005; Stout et al, 1999). As expected, time of exposure to the cold pressor did predict the hormone response (Lovallo, 1975), but this too was similar across groups and was, in fact, nonsignificantly briefer in the hyponatremic polydipsic subjects (Table 2). Posm was normalized before the cold pressor (Table 2), so that concurrent hypoosmolemia/hyponatremia per se can be excluded. Pre-infusion osmolality was negatively correlated with the AVP response, consistent with the recognized blunting effects of chronic hyponatremia on AVP and ACTH responses, (Dohanics et al, 1991; Goldman et al, 1996; Robertson, 2001). Thus the role of long-standing hyponatremia can also be discounted, though inclusion of a non-psychiatric control group with hyponatremia would more definitively address this possibility. Polydipsia cannot account for the findings, as it too blunts AVP responses (Moses and Clayton, 1993) and in any event, the PNS patients' responses were blunted relative to those with hyponatremia (Figure 2a and b). Finally, the diminished blood pressure response in NNS patients cannot explain their blunted neuroendocrine responses, as blood pressure change tends to be inversely correlated with the neuroendocrine responses (al'Absi and Petersen, 2003).
Responses Suggestive of Altered Response to Psychological Stress
Although more definitive conclusions require replication with other sets of stimuli, these data support the concept that responses to psychological, but not systemic stress, diverge in schizophrenia depending on the presence and severity of water imbalance. Thus, previous studies indicate the cold pressor has the attributes of a psychological stressor (eg aversiveness and productive of anticipatory responses), and that these tend to increase HPA activity (al'Absi and Petersen, 2003; Bullinger et al, 1984; Ehrenreich et al, 1997; Gregg et al, 1999), whereas its systemic effects (eg cold, hypertension) tend to decrease activity (Edelson and Robertson, 1986; Kendler et al, 1978; Wittert et al, 1992). Group responses to a systemic stimulus (Hennig et al, 2000; Jacob et al, 1998), which produced an equivalent HPA and perhaps greater AVP response (compare Figure 2 and Supplementary Figure 2), were similar. We must acknowledge, however, that although the effects of the systemic stimulus in normals is attributable to its systemic actions, we cannot exclude the possibility that psychological factors contribute to its actions in persons with schizophrenia.
Mechanism of Water Intoxication in Schizophrenia
Impaired water excretion that varies with the stress of acute psychosis was first demonstrated before the introduction of antipsychotic medications by Targowla (1923), and subsequently confirmed in medication naive patients by Hobson and English (1963) and Suzuki et al (1992). Barahal in 1938 first noted the association between acute psychosis and life-threatening water intoxication (also see de Leon et al, 1994). Subsequent studies in controlled experimental settings have demonstrated that both the basal impairments in water excretion (Goldman et al, 1988, 1996) and their aggravation during acute psychosis (Goldman et al, 1997) are attributable to unexplained increases in vasopressin activity that are not seen in other schizophrenic patients. Although effective treatments for hyponatremia have subsequently been introduced, reports of fatalities from water intoxication continue to accumulate (Farrell and Bower, 2003; Hayashi et al, 2005; Loas and Mercier-Guidez, 2002). The current study establishes a plausible mechanism for the previously unexplained rise in vasopressin activity in hyponatremic schizophrenics: an enhanced neuroendocrine response to psychological stress.
Model of Enhanced Stress Vulnerability
Although the mechanism is not apparent from these data, accumulating evidence indicates anterior hippocampal formation pathology may be responsible for this enhanced neuroendocrine activity. Thus this segment of the hippocampus normally constrains AVP and ACTH responses to psychological stress (Herman et al, 1995, 2005; Mueller et al, 2004; Nettles et al, 2000); the findings in patients are recreated in an animal model of schizophrenia, which disrupts the neurodevelopment of this brain region (Mitchell and Goldman, 2004); and this subset of patients exhibits diminished anterior hippocampal volume (Goldman et al, 2005) as well as other impairments indicative of hippocampal-mediated neuroendocrine dysfunction (Goldman et al, 2006). Finally, our unpublished data demonstrate a significant association between anterior hippocampal volume and the cold pressor AVP response in hyponatremic polydipsic patients (r=−0.825, p=0.022). More direct evidence is needed, however, before rejecting alternative explanations.
A hippocampal-mediated vulnerability to psychological stress has been proposed to underlie schizophrenia (Mednick and Schulsinger, 1968; Gray, 1998; Walker and Diforio, 1997; Moghaddam, 2002; Myin-Germeys et al, 2005), and thus PHS schizophrenic patients may facilitate our understanding of how hippocampal pathology increases stress vulnerability. This region of the hippocampus has been associated not only with schizophrenia and neuroendocrine responses to psychological stress but also with affective and behavioral stress responses (Flores et al, 2005; Trivedi and Coover, 2004), suggesting the neuroendocrine disorder could be a manifestation of the pathologic changes responsible for the severe mental illness.
Strengths, Caveats, and Limitations
The strengths of this study are the inclusion of the two matched control groups of schizophrenic patients; the two-stage selection process to characterize water balance in the patient groups; the acclimation of subjects to the research setting; the saline infusion to normalize Posm; the inclusion of a control (systemic) stimulus; the characterization of the normal cold pressor response in the HC; and the careful monitoring of possible confounding factors in a clinical research center.
There are, however, important caveats and limitations. Thus, although the sample size is similar to those of previously reported studies (Goldman et al, 1988, 1997), it is small, thereby suggesting findings may be difficult to reproduce or that undetected group differences (ie type II errors) could account for them. The cold pressor is a mixed physical and psychological stimulus, and although our data and that of others suggest that the neuroendocrine release is attributable to its psychological effects, we cannot exclude that group differences in systemic responses contribute to the results. Also we cannot exclude the possibility that the cold pressor altered the impact of the subsequent postural stimulus.
Finally, the findings do not address the mechanism of the blunted neuroendocrine responses in patients with normal water balance. Furthermore, although they replicate previous observations of diminished blood pressor response to cold pressor in these patients (Earle and Earle, 1955), the data (Table 2) appear inconsistent with the broad literature suggestive of pain insensitivity in schizophrenia (Singh et al, 2006). Further studies are required to resolve these issues.
References
al'Absi M, Petersen KL (2003). Blood pressure but not cortisol mediates stress effects on subsequent pain perception in healthy men and women. Pain 106: 285–295.
Albus M, Ackenheil M, Engel RR, Muller F (1982). Situational reactivity of autonomic functions in schizophrenic patients. Psychiatry Res 6: 361–370.
Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T et al (2004). Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev 28: 273–283.
Barahal HS (1938). Water intoxication in a mental case. Psychiatr Q 12: 767–771.
Barbas H, Blatt GJ (1995). Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus 5: 511–533.
Bech P, Kastrup M, Rafaelsen OJ (1986). Mini-compendium of rating scales for states of anxiety, depression, mania, and schizophrenia with corresponding DSM-III syndromes. Acta Psychiatr Scand 73 (Suppl 326): 7–37.
Braehler C, Holowka D, Brunet A, Beaulieu S, Baptista T, Debruille JB et al (2005). Diurnal cortisol in schizophrenia patients with childhood trauma. Schizophr Res 79: 353–354.
Breier A, Wolkowitz OM, Doran AR, Bellar S, Pickar D (1988). Neurobiological effects of lumbar puncture stress in psychiatric patients and healthy volunteers. Psychiatry Res 25: 187–194.
Bullinger M, Naber D, Pickar D, Cohen RM, Kalin NH, Pert A et al (1984). Endocrine effects of the cold pressor test: relationships to subjective pain appraisal and coping. Psychiatry Res 12: 227–233.
Chrapusta SJ, Egan MF, Wyatt RJ, Weinberger DR, Lipska BK (2003). Neonatal ventral hippocampal damage modifies serum corticosterone and dopamine release responses to acute footshock in adult Sprague–Dawley rats. Synapse 47: 270–277.
Dawson ME, Nuechterlein KH (1984). Psychophysiological dysfunctions in the developmental course of schizophrenic disorders. Schizophr Bull 10: 204–232.
de Leon J, Verghese C, Tracy JI, Josiassen RC, Simpson GM (1994). Polydipsia and water intoxication in psychiatric patients: a review of the epidemiological literature. Biol Psychiatry 35: 519–530.
Dohanics J, Hoffman GE, Verbalis JG (1991). Hyponatremia-induced inhibition of magnocellular neurons causes stressor-selective impairment of stimulated adrenocorticotropin secretion in rats. Endocrinology 128: 331–340.
Earle A, Earle BV (1955). The blood pressure response to pain and emotion in schizophrenia. J Nerv Ment Dis 121: 132–139.
Edelson JT, Robertson GL (1986). The effect of the cold pressor test on vasopressin secretion in man. Psychoneuroendocrinology 11: 307–316.
Ehrenreich H, Dieck K, Gefeller O, Kaw S, Schilling L, Poser W et al (1997). Sustained elevation of vasopressin plasma levels in healthy young men, but not in abstinent alcoholics, upon expectation of novelty. Psychoneuroendocrinology 22: 13–24.
Elkashef AM, Leadbetter RA, Kirch DG (1996). Structural brain imaging in patients with schizophrenia and the syndrome of polydipsia-hyponatremia. In: Schnur D, Kirch DG (eds). Water Balance in Schizophrenia. American Psychiatric Press: Washington, DC. pp 125–136.
Elman I, Adler CM, Malhotra AK, Bir C, Pickar D, Breier A (1998). Effect of acute metabolic stress on pituitary–adrenal axis activation in patients with schizophrenia. Am J Psychiatry 155: 979–981.
Endicott J, Spitzer R, Fleiss J, Cohen J (1976). The Global Assessment Scale: A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 33: 767–771.
Farhadi A, Keshavarzian A, Van de Kar LD, Jakate S, Domm A, Zhang L et al (2005). Heightened responses to stressors in patients with inflammatory bowel disease. Am J Gastroenterol 100: 1796–1804.
Farrell DJ, Bower L (2003). Fatal water intoxication. J Clin Pathol 56: 803–804.
First MB, Spitzer RL, Gibbon M, Williams JBW (1996). Structured Clinical Interview for DSM-IV Axis I Disorders-Non Patient Edition. Biometrics Research Department, New York State Psychiatric Institute: New York.
Flores G, Silva-Gomez AB, Barbeau D, Srivastava LK, Zamudio S, De La Cruz Lopez F (2005). Effect of excitotoxic lesions of the neonatal ventral hippocampus on the immobility response in rats. Life Sci 76: 2339–2348.
Gaab J, Rohleder N, Nater UM, Ehlert U (2005). Psychological determinants of the cortisol stress response: the role of anticipatory cognitive appraisal. Psychoneuroendocrinology 30: 599–610.
Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB et al (1993). Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Application to the NIMH treatment of Depression Collaborative Research Program dataset. Arch Gen Psychiatry 50: 739–750.
Gispen-de Wied CC (2000). Stress in schizophrenia: an integrative view. Eur J Pharmacol 405: 375–384.
Goldman MB, Blake L, Marks RC, Hedeker D, Luchins DJ (1993). Association of nonsuppression of cortisol on the DST with primary polydipsia in chronic schizophrenia. Am J Psychiatry 150: 653–655.
Goldman MB, Hussain N (2004). Absence of effect of olanzapine on primary polydipsia: results of a double-blind randomized study. J Clin Psychopharmacol 24: 678–680.
Goldman MB, Hussain H, Wood G, Goldman MB, Gavin M, Paul S et al (2006). Mechanism of impaired corticosteroid negative feedback in schizophrenia (online: doi:10.1210/jc.2006-1131 J Clin Endocrinol Metab).
Goldman MB, Luchins DJ, Robertson GL (1988). Mechanisms of altered water metabolism in psychotic patients with polydipsia and hyponatremia. N Eng J Med 318: 397–403.
Goldman MB, Robertson GL, Luchins DJ, Hedeker D (1996). The influence of polydipsia on water excretion in hyponatremic, polydipsic schizophrenic patients. J Clin Endocrinol Metab 81: 1465–1470.
Goldman MB, Robertson GL, Luchins DJ, Hedeker D, Pandey GN (1997). Psychotic exacerbations and enhanced vasopressin secretion in schizophrenics with hyponatremia and polydipsia. Arch Gen Psychiatry 54: 443–449.
Goldman MB, Torres IJ, Keedy S, Marlow-O'Connor M, Beenken B (2005). Reduced anterior hippocampal volume in patients with schizophrenia and water imbalance. Schizo Bull 31: 398 (abstract).
Gray JA (1998). Integrating schizophrenia. Schizophr Bull 24: 249–266.
Gregg ME, James JE, Matyas TA, Thorsteinsson EB (1999). Hemodynamic profile of stress-induced anticipation and recovery. Int J Psychophysiol 34: 147–162.
Hayashi T, Ishida Y, Miyashita T, Kiyokawa H, Kimura A, Kondo T (2005). Fatal water intoxication in a schizophrenic patient—an autopsy case. J Clin Forensic Med 12: 157–159.
Hedeker D, Gibbons RD (1996a). MIXREG: a computer program for mixed-effects regression analysis with autocorrelated errors. Comput Methods Programs Biomed 49: 229–252.
Hedeker D, Gibbons RD (2006). Longitudinal Data Analysis. Wiley: Hoboken, NJ.
Hedeker D, Gibbons RD, MIXOR (1996b). MIXOR: a computer program for mixed-effects ordinal regression analysis. Comput Methods Programs Biomed 49: 157–176.
Hennig J, Friebe J, Ryl I, Kramer B, Bottcher J, Netter P (2000). Upright posture influences salivary cortisol. Psychoneuroendocrinology 25: 69–83.
Herman JP, Cullinan WE, Morano MI, Akil H, Watson SJ (1995). Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo–pituitary–adrenocortical axis. J Neuroendocrinol 7: 475–482.
Herman JP, Ostrander MM, Mueller NK, Figueiredo H (2005). Limbic system mechanisms of stress regulation: hypothalamo–pituitary–adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29: 1201–1213.
Herman JP, Tasker JG, Ziegler DR, Cullinan WE (2002). Local circuit regulation of paraventricular nucleus stress integration glutamate-GABA connections. Pharm Biochem Behav 71: 457–468.
Hobson JA, English JT (1963). Self-induced water intoxication: case study of a chronically schizophrenic patient with physiological evidence of water retention due to inappropriate release of antidiuretic hormone. Ann Intern Med 58: 324–332.
Hodes RL, Howland EW, Lightfoot N, Cleeland CS (1990). The effects of distraction on responses to cold pressor pain. Pain 41: 109–114.
Jacob G, Ertl AC, Shannon JR, Furlan R, Robertson RM, Robertson D (1998). Effect of standing on neurohumoral responses and plasma volume in healthy subjects. J Appl Physiol 84: 914–921.
Jansen LM, Gispen-de Wied CC, Gademan PJ, De Jonge RC, van der Linden JA, Kahn RS (1998). Blunted cortisol response to a psychosocial stressor in schizophrenia. Schizophr Res 33: 87–94.
Jansen LM, Gispen-de Wied CC, Kahn RS (2000). Selective impairments in the stress response in schizophrenic patients. Psychopharmacology (Berlin) 149: 319–325.
Kaneda Y, Fujii A, Ohmori T (2002). The hypothalamic–pituitary–adrenal axis in chronic schizophrenic patients long-term treated with neuroleptics. Prog Neuropsychopharmacol Biol Psychiatry 26: 935–938.
Kawai N, Baba A, Suzuki T (2002). Risperidone failed to improve polydipsia-hyponatremia of the schizophrenic patients. Psychiatry Clin Neurosci 56: 107–110.
Kay SR, Fiszbein S, Opler LA (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 113: 261–276.
Kendler KS, Weitzman RE, Fisher DA (1978). The effect of pain on plasma arginine vasopressin concentrations in man. Clin Endocrinol (Oxford) 8: 89–94.
Kudoh A, Ishihara H, Matsuki A (1999). Pituitary–adrenal and parasympathetic function in chronic schizophrenic patients with postoperative ileus or hypotension. Neuropsychobiology 39: 125–130.
Lipska BK (2004). Using animal models to test a neurodevelopmental hypothesis of schizophrenia. Psychiatry Neurosci 29: 282–286.
Loas G, Mercier-Guidez E (2002). Fatal self-induced water intoxication among schizophrenic inpatients. Eur Psychiatry 17: 307–310.
Lopes-Machado EZ, Crippa JA, Hallak JE, Guimaraes FS, Zuardi AW (2002). Electrodermically nonresponsive schizophrenia patients make more errors in the Stroop Color Word Test, indicating selective attention deficit. Schizophr Bull 28: 459–466.
Lovallo W (1975). The cold pressor test and autonomic function: a review and integration. Psychophysiology 12: 268–282.
Luchins DJ (1990). A possible role of hippocampal dysfunction in schizophrenic symptomatology. Biol Psychiatry 28: 87–91.
Luchins DJ, Nettles KW, Goldman MB (1997). Anterior medial temporal lobe volumes in polydipsic schizophrenic patients with and without hypo-osmolemia: A pilot study. Biol Psychiatry 42: 767–770.
Mann K, Rossbach W, Muller MJ, Muller-Siecheneder F, Pott T, Linde I et al (2006). Nocturnal hormone profiles in patients with schizophrenia treated with olanzapine. Psychoneuroendocrinology 31: 256–264.
Marcelis M, Cavalier E, Gielen J, Delespaul P, Van Os J (2004). Abnormal response to metabolic stress in schizophrenia: marker of vulnerability or acquired sensitization? Psychol Med 34: 1103–1111.
Mednick S, Schulsinger F (1968). Some premorbid characteristics related to breakdown in children with schizophrenic mothers. In: Rosenthal D, Kety S (eds). The Transmission of Schizophrenia. Pergamon: Oxford. pp 267–293.
Mitchell CP, Goldman MB (2004). Neonatal lesions of the ventral hippocampal formation disrupt neuroendocrine responses to auditory stress in the adult rat. Psychoneuroendocrinology 29: 1317–1325.
Moghaddam B (2002). Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry 51: 775–787.
Moser MB, Moser EI (1998). Functional differentiation in the hippocampus. Hippocampus 8: 608–619.
Moses AM, Clayton B (1993). Impairment of osmotically stimulated AVP release in patients with primary polydipsia. Am J Physiol 265: R1247–R1252.
Mueller NK, Dolgas CM, Herman JP (2004). Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses. Endocrinology 45: 3763–3768.
Myin-Germeys I, Delespaul P, van Os J (2005). Behavioural sensitization to daily life stress in psychosis. Psychol Med 35: 733–741.
Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP et al (2004). Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage 21: 1563–1575.
Nettles KW, Pesold C, Goldman MB (2000). Influence of the ventral hippocampal formation on plasma vasopressin, hypothalamic–pituitary–adrenal axis, and behavioral responses to novel acoustic stress. Brain Res 858: 181–190.
Onaka T, Yagi K (1998). Role of noradrenergic projections to the bed nucleus of the stria terminalis in neuroendocrine and behavioral responses to fear-related stimuli in rats. Brain Res 788: 287–293.
Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC (2005). A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology 30: 80–91.
Pegues MP, Rogers LJ, Amend D, Vinogradov S, Deicken RF (2003). Anterior hippocampal volume reduction in male patients with schizophrenia. Schizophr Res 60: 105–115.
Peters ML, Godaert GL, Ballieux RE, van Vliet M, Willemsen JJ, Sweep FC et al (1998). Cardiovascular and endocrine responses to experimental stress: effects of mental effort and controllability. Psychoneuroendocrinology 23: 1–17.
Risold PY, Swanson LW (1996). Structural evidence for functional domains in the rat hippocampus. Science 272: 1484–1486.
Robertson GL (2001). Antidiuretic hormone. Normal and disordered function. Endocrinol Metab Clin N Am 30: 671–694.
Rubin RT, Mandell AJ, Crandall PH (1966). Corticosteroid responses to limbic stimulation in man: localization of stimulus sites. Science 153: 767–768.
Shutty Jr MS, Leadbetter RA, McCulley K (1997). Diurnal urine volume estimates in schizophrenic patients with polydipsia during water-loaded versus nonloaded states. Biol Psychiatry 41: 374–376.
Singh MK, Giles LL, Nasrallah HA (2006). Pain insensitivity in schizophrenia: trait or state marker. J Psychol Pract 12: 90–102.
Spitzer RL, Williams JBW, Gibbon M, First MB (1992). The Structured Clinical Interview for DSM-III-R : History, rationale and description. Arch Gen Psychiatry 49: 624–629.
Stout NR, Kenny RA, Baylis PH (1999). A review of water balance in ageing in health and disease. Gerontology 45: 61–66.
Strange BA, Dolan RJ (2001). Adaptive anterior hippocampal responses to oddball stimuli. Hippocampus 11: 690–698.
Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ (1999). Segregating the functions of human hippocampus. Proc Natl Acad Sci USA 96: 4034–4039.
Suzuki M, Takeuchi O, Mori I, Takegoshi K, Kurachi M (1992). Syndrome of inappropriate secretion of antidiuretic hormone associated with schizophrenia. Biol Psychiatry 31: 1057–1061.
Szeszko PR, Goldberg E, Gunduz-Bruce H, Ashtari M, Robinson D, Malhotra AK et al (2003). Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. Am J Psychiatry 160: 2190–2197.
Targowla R (1923). Des troubles fonctionnel du rein dans les maladies mentales. L'excretion del'eau. Bull Soc Med Hop Paris 47: 1711–1715.
Trivedi MA, Coover GD (2004). Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiol Learn Mem 81: 172–184.
Tuvnes FA, Steffenach HA, Murison R, Moser MB, Moser EI (2003). Selective hippocampal lesions do not increase adrenocortical activity. J Neurosci 23: 4345–4354.
van Eekelen AP, Kerkhof GA, van Amsterdam JG (2003). Circadian variation in cortisol reactivity to an acute stressor. Chronobiol Int 20: 863–878.
Velakoulis D, Stuart GW, Wood SJ, Smith DJ, Brewer WJ, Desmond P et al (2001). Selective bilateral hippocampal volume loss in chronic schizophrenia. Biol Psychiatry 50: 531–539.
Walder DJ, Walker EF, Lewine RJ (2000). Cognitive functioning, cortisol release, and symptom severity in patients with schizophrenia. Biol Psychiatry 48: 1121–1132.
Walker EF, Diforio D (1997). Schizophrenia: a neural diathesis-stress model. Psychol Rev 104: 667–685.
Walsh P, Spelman L, Sharifi N, Thakore JH (2005). Male patients with paranoid schizophrenia have greater ACTH and cortisol secretion in response to metoclopramide-induced AVP release. Psychoneuroendocrinology 30: 431–437.
Weiss AP, Dewitt I, Goff D, Ditman T, Heckers S (2005). Anterior and posterior hippocampal volumes in schizophrenia. Schizophr Res 73: 103–112.
Wittert GA, Or HK, Livesey JH, Richards AM, Donald RA, Espiner EA (1992). Vasopressin, corticotrophin-releasing factor, and pituitary adrenal responses to acute cold stress in normal humans. J Clin Endocrinol Metab 75: 750–755.
Zahn TP, Pickar D (2005). Autonomic activity in relation to symptom ratings and reaction time in unmedicated patients with schizophrenia. Schizophr Res 79: 257–270.
Acknowledgements
We are indebted to the patients and staff at the Psychiatric Clinical Research Center, University of Illinois at Chicago, Chicago, IL; to the nursing, laboratory and support staffs of the Clinical Research Centers at University of Illinois at Chicago and the University of Chicago; to Sheila Dowd, PhD, Jane Strong, RN and Beth Winans, PhD for completing ratings and working closely with staff and patients; to Barbara Brown, MS; Mary Beth Gaskill, BA; Colin Mitchell, PhD, Neil Scherberg, PhD, Ghazala Fayyaz, MD, Stacey Paul, BS, Suhaila Zaheer, MD, MA, Javaid Shah, MD, Megan Goldman, BA, for their outstanding technical assistance; and to Paul Feldman, MD, Dan Luchins, MD, Phil Janicak, MD, Gary L Robertson, MD, Brian Kirkpatrick, MD, Eve Van Cauter, PhD, Robert Gibbons, PhD and Roy Weiss, MD, PhD for assistance with design of the studies or reviews of the paper. Funding provided by Brain Research Foundation, an affiliate of the University of Chicago; State of Illinois funding of the Psychiatric Institute; NIH: RO1 MH56525 (MG), General Clinical Research Center Grants: M01 RR00055 (UC), M01-RR-13987 (UIC). Dr Goldman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Rights and permissions
About this article
Cite this article
Goldman, M., Gnerlich, J. & Hussain, N. Neuroendocrine Responses to a Cold Pressor Stimulus in Polydipsic Hyponatremic and in Matched Schizophrenic Patients. Neuropsychopharmacol 32, 1611–1621 (2007). https://doi.org/10.1038/sj.npp.1301282
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301282
Keywords
This article is cited by
-
Haloperidol affects bones while clozapine alters metabolic parameters - sex specific effects in rats perinatally treated with phencyclidine
BMC Pharmacology and Toxicology (2017)
-
Polidipsia primaria: è realmente una condizione di raro riscontro nella pratica clinica?
L'Endocrinologo (2016)
-
Schedule-induced polydipsia as a model of compulsive behavior: neuropharmacological and neuroendocrine bases
Psychopharmacology (2012)
-
L’indifférence à la douleur dans la schizophrénie
Douleur et Analgésie (2011)
-
Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia
Psychopharmacology (2011)