Abstract
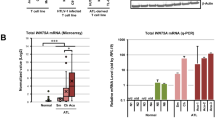
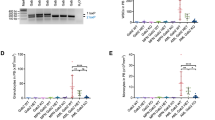
Transforming growth factor-β (TGF-β)-stimulated clone-22 (TSC-22) was originally isolated as a TGF-β-inducible gene. In this study, we identified TSC-22 as a potential leukemia suppressor. Two types of FMS-like tyrosine kinase-3 (Flt3) mutations are frequently found in acute myeloid leukemia: Flt3-ITD harboring an internal tandem duplication in the juxtamembrane domain associated with poor prognosis and Flt3-TKD harboring a point mutation in the kinase domain. Comparison of gene expression profiles between Flt3-ITD- and Flt3-TKD-transduced Ba/F3 cells revealed that constitutive activation of Flt3 by Flt3-TKD, but not Flt3-ITD, upregulated the expression of TSC-22. Importantly, treatment with an Flt3 inhibitor PKC412 or an Flt3 small interfering RNA decreased the expression level of TSC-22 in Flt3-TKD-transduced cells. Forced expression of TSC-22 suppressed the growth and accelerated the differentiation of several leukemia cell lines into monocytes, in particular, in combination with differentiation-inducing reagents. On the other hand, a dominant-negative form of TSC-22 accelerated the growth of Flt3-TKD-transduced 32Dcl.3 cells. Collectively, these results suggest that TSC-22 is a possible target of leukemia therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Matthews W, Jordan CT, Wiegand GW, Pardoll D, Lemischka IR . A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell 1991; 65: 1143–1152.
Rosnet O, Marchetto S, deLapeyriere O, Birnbaum D . Oncogene 1991; 6: 1641–1650.
Small D, Levenstein M, Kim E, Carow C, Amin S, Rockwell P et al. STK-1, the human homolog of Flk-2/Flt3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci USA 1994; 91: 459–463.
Rosnet O, Buhring HJ, Marchetto S, Rappold I, Lavagna C, Sainty D et al. Human FLT3/FLK2 receptor tyrosine kinase is expressed at the surface of normal and malignant hematopoietic cells. Leukemia 1996; 10: 238–248.
Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR . Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity 1995; 3: 147–161.
Birg F, Courcoul M, Rosnet O, Bardin F, Pebusque MJ, Marchetto S et al. Expression of the FMS/KIT-like gene FLT3 in human acute leukemias of the myeloid and lymphoid lineages. Blood 1992; 80: 2584–2593.
Carow CE, Levenstein M, Kaufmann SH, Chen J, Amin S, Rockwell P et al. Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood 1996; 87: 1089–1096.
Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K et al. Internal tandem duplication of the Flt3 gene found in acute myeloid leukemia. Leukemia 1996; 10: 1911–1918.
Kiyoi H, Towatari M, Yokota S, Hamaguchi M, Ohno R, Saito H et al. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia 1998; 12: 1333–1337.
Meshinchi S, Woods WG, Stirewalt DL, Sweetser DA, Buckley JD, Tjoa TK et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood 2001; 97: 89–94.
Stirewalt DL, Kopecky KJ, Meshinchi S, Appelbaum FR, Slovak ML, Willman CL et al. FLT3, RAS and TP53 mutations in elderly patients with acute myeloid leukemia. Blood 2001; 97: 3589–3595.
Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood 2002; 100: 59–66.
Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002; 99: 4326–4335.
Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood 2001; 97: 2434–2439.
Abu-Duhier FM, Goodeve AC, Wilson GA, Care RS, Peake IR, Reilly JT . Identification of novel FLT3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol 2001; 113: 983–988.
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA et al. The presence of a FLT3 internal tandem duplication in pa-tients with acute myeloid leukemia (AML) addsimportant prognostic information to cytogeneticrisk group and response to the first cycle of che-motherapy: analysis of 854 patients from theUnited Kingdom Medical Research Council AML10 and 12 trials. Blood 2001; 98: 1752–1759.
Hayakawa F, Towatari M, Kiyoi H, Tanimoto M, Kitamura T, Saito H et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene 2000; 19: 624–631.
Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Muller C et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood 2000; 96: 3907–3914.
Fenski R, Flesch K, Serve S, Mizuki M, Oelmann E, Kratz-Albers K et al. Constitutive activation of FLT3 in acute myeloid leukaemia and its consequences for growth of 32D cells. Br J Haematol 2000; 108: 322–330.
Stirewalt DL, Radich JP . The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer 2003; 3: 650–665.
Shih LY, Huang CF, Wu JH, Lin TL, Dunn P, Wang PN et al. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: a comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood 2002; 100: 2387–2392.
Moreno I, Martin G, Bolufer P, Barragan E, Rueda E, Roman J et al. Incidence and prognostic value of FLT3 internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica 2003; 88: 19–24.
Levis M, Small D . FLT3: ITDoes matter in leukemia. Leukemia 2003; 17: 1738–1752.
Spiekermann K, Bagrintseva K, Schwab R, Schmieja K, Hiddemann W . Overexpression and constitutive activation of FLT3 induces STAT5 activation in primary acute myeloid leukemia blast cells. Clin Cancer Res 2003; 9: 2140–2150.
Choudhary C, Schwable J, Brandts C, Tickenbrock L, Sargin B, Kindler T et al. AML-associated Flt3 kinase domain mutations show signal transduction differences compared with Flt3 ITD mutations. Blood 2005; 106: 265–273.
Shibanuma M, Kuroki T, Nose K . Isolation of a gene encoding a putative leucine zipper structure that is induced by transforming growth factor beta 1 and other growth factors. J Biol Chem 1992; 267: 10219–10224.
Ohta S, Shimekake Y, Nagata K . Molecular cloning and characterization of a transcription factor for the C-type natriuretic peptide gene promoter. Eur J Biochem 1996; 242: 460–466.
Jay P, Ji JW, Marsollier C, Taviaux S, Berge-Lefranc JL, Berta P . Cloning of the human homologue of the TGF beta-stimulated clone 22 gene. Biochem Biophys Res Commun 1996; 222: 821–826.
Kawamata H, Nakashiro K, Uchida D, Hino S, Omotehara F, Yoshida H et al. Induction of TSC-22 by treatment with a new anti-cancer drug, vesnarinone, in a human salivary gland cancer cell. Br J Cancer 1998; 77: 71–78.
Hamil KG, Hall SH . Cloning of rat Sertoli cell follicle-stimulating hormone primary response complementary deoxyribonucleic acid: regulation of TSC-22 gene expression. Endocrinology 1994; 134: 1205–1212.
Kawa-uchi T, Nifuji A, Mataga N, Olson EN, Bonaventure J, Shinomiya K et al. Fibroblast growth factor downregulates expression of a basic helix-loop-helix-type transcription factor, scleraxis, in a chondrocyte-like cell line, TC6. J Cell Biochem 1998; 70: 468–477.
Kester HA, van der Leede BM, van der Saag PT, van der Burg B . Novel progesterone target genes identified by an improved differential display technique suggest that progestin-induced growth inhibition of breast cancer cells coincides with enhancement of differentiation. J Biol Chem 1997; 272: 16637–16643.
Trenkle T, Welsh J, Jung B, Mathieu-Daude F, McClelland M . Non-stoichiometric reduced complexity probes for cDNA arrays. Nucleic Acids Res 1998; 26: 3883–3891.
Dohrmann CE, Belaoussoff M, Raftery LA . Dynamic expression of TSC-22 at sites of epithelial-mesenchymal interactions during mouse development. Mech Dev 1999; 84: 147–151.
Kester HA, Ward-van Oostwaard TM, Goumans MJ, van Rooijen MA, van Der Saag PT, van Der Burg B et al. Expression of TGF-beta stimulated clone-22 (TSC-22) in mouse development and TGF-beta signalling. Dev Dyn 2000; 218: 563–572.
Ohta S, Yanagihara K, Nagata K . Mechanism of apoptotic cell death of human gastric carcinoma cells mediated by transforming growth factor beta. Biochem J 1997; 324: 777–782.
Nakashiro K, Kawamata H, Hino S, Uchida D, Miwa Y, Hamano H et al. Down-regulation of TSC-22 (transforming growth factor beta-stimulated clone 22) markedly enhances the growth of a human salivary gland cancer cell line in vitro and in vivo. Cancer Res 1998; 58: 549–555.
Hino S, Kawamata H, Uchida D, Omotehara F, Miwa Y, Begum NM et al. Nuclear translocation of TSC-22 (TGF-beta-stimulated clone-22) concomitant with apoptosis: TSC-22 as a putative transcriptional regulator. Biochem Biophys Res Commun 2000; 278: 659–664.
Murata K, Kumagai H, Kawashima T, Tamitsu K, Irie M, Nakajima H et al. Selective cytotoxic mechanism of GTP-14564, a novel tyrosine kinase inhibitor in leukemia cells expressing a constitutively active Fms-like tyrosine kinase 3 (FLT3). J Biol Chem 2003; 278: 32892–32898.
Kitamura T, Koshino Y, Shibata F, Oki T, Nakajima H, Nosaka T et al. Retrovirus-mediated gene transfer and expression cloning powerful tools in functional genomics. Exp Hematol 2003; 31: 1007–1014.
Oki T, Kitaura J, Eto K, Lu Y, Maeda-Yamamoto M, Inagaki N et al. Integrin alphaIIbbeta3 induces the adhesion and activation of mast cells through interaction with fibrinogen. J Immunol 2006; 76: 52–60.
Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell 2002; 1: 433–443.
Walters DK, Stoffregen EP, Heinrich MC, Deininger MW, Druker BJ . RNAi-induced down-regulation of FLT3 expression in AML cell lines increases sensitivity to MLN518. Blood 2005; 105: 2952–2954.
Kester HA, Blanchetot C, den Hertog J, van der Saag PT, van der Burg B . Transforming growth factor-beta-stimulated clone-22 is a member of a family of leucine zipper proteins that can homo- and heterodimerize and has transcriptional repressor activity. J Biol Chem 1999; 274: 27439–27447.
Zheng R, Friedman AD, Small D . Targeted inhibition of FLT3 overcomes the block to myeloid differentiation in 32Dcl3 cells caused by expression of FLT3/ITD mutations. Blood 2002; 100: 4154–4161.
Shostak KO, Dmitrenko VV, Garifulin OM, Rozumenko VD, Khomenko OV, Zozulya YA et al. Downregulation of putative tumor suppressor gene TSC-22 in human brain tumors. J Surg Oncol 2003; 82: 57–64.
Rentsch CA, Cecchini MG, Schwaninger R, Germann M, Markwalder R, Heller M et al. Differential expression of TGF beta-stimulated clone 22 in normal prostate and prostate cancer. Int J Cancer 2006; 118: 899–906.
Mizuki M, Schwable J, Steur C, Choudhary C, Agrawal S, Sargin B et al. Suppression of myeloid transcription factors and induction of STAT response genes by AML-specific Flt3 mutations. Blood 2003; 101: 3164–3173.
Uchida D, Kawamata H, Omotehara F, Miwa Y, Hino S, Begum NM et al. Over-expression of TSC-22 (TGF-beta stimulated clone-22) markedly enhances 5-fluorouracil-induced apoptosis in a human salivary gland cancer cell line. Lab Invest 2000; 80: 955–963.
Choi SJ, Moon JH, Ahn YW, Ahn JH, Kim DU, Han TH . Tsc-22 enhances TGF-beta signaling by associating with Smad4 and induces erythroid cell differentiation. Mol Cell Biochem 2005; 271: 23–28.
Glasow A, Prodromou N, Xu K, von Lindern M, Zelent A . Retinoids and myelomonocytic growth factors cooperatively activate RARA and induce human myeloid leukemia cell differentiation via MAP kinase pathways. Blood 2005; 105: 341–349.
Acknowledgements
We thank Dr Hitoshi Kawamata for providing plasmid pEGFP-TSC-22-LZ. We thank Dr Naoko Watanabe, Dr Yoshinori Yamanishi, Dr Kumi Izawa and Dr Yukinori Minoshima for valuable discussions, Fumi Shibata, Miyuki Ito and Ai Hishiya for excellent technical assistance. We also thank R&D Systems for cytokines. This work was supported in part by Grants-in-Aid from the Ministry of Education, Science, Technology, Sports, and Culture in Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Y., Kitaura, J., Oki, T. et al. Identification of TSC-22 as a potential tumor suppressor that is upregulated by Flt3-D835V but not Flt3-ITD. Leukemia 21, 2246–2257 (2007). https://doi.org/10.1038/sj.leu.2404883
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404883
Keywords
This article is cited by
-
RNA-binding protein MEX3D promotes cervical carcinoma tumorigenesis by destabilizing TSC22D1 mRNA
Cell Death Discovery (2022)
-
Aberrant expression of RasGRP1 cooperates with gain-of-function NOTCH1 mutations in T-cell leukemogenesis
Leukemia (2012)
-
Regulation of TGF-β signaling by PKC depends on Tsc-22 inducibility
Molecular and Cellular Biochemistry (2012)
-
Differential signaling of Flt3 activating mutations in acute myeloid leukemia: a working model
Protein & Cell (2011)