Abstract
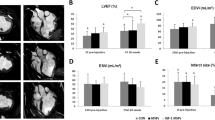
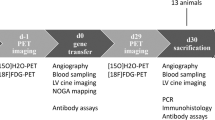
Gelatin hydrogel microspheres (GHMs) have been reported as novel non-viral vectors for gene or protein delivery (GHM therapy). However, the components of an effective catheter-based delivery strategy for GHM therapy are unknown. We evaluated the effectiveness of three catheter-based strategies for cardiac GHM therapy: (1) antegrade injection (AI) via coronary arteries; (2) retrograde injection (RI) via coronary veins; and (3) direct myocardial injection (DI) via the coronary sinus. AI distributed microspheres homogeneously throughout the target area with 73±11% retention. RI scattered microspheres non-homogenously with 22±8% retention. DI distributed microspheres in the needle-advanced area with 47±14% retention. However, despite high efficiency, AI did not show biological effects of inducing angiogenesis from basic fibroblast growth factor bound to GHMs. Furthermore, focal micro-infarctions, owing to micro-embolism of aggregated GHMs into small coronary arterioles, were detected in the AI group. Conversely, only RI and DI groups displayed increased coronary flow reserve. DI groups also demonstrated increased capillary density. These results suggest that RI and DI are effective for cardiac GHM therapy, while AI appears inappropriate owing to the risk of focal infarctions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med 2002; 347: 1397–1402.
Hajjar RJ, del Monte F, Matsui T, Rosenzweig A . Prospects for gene therapy for heart failure. Circ Res 2000; 86: 616–621.
Donahue JK, Heldman AW, Fraser H, McDonald AD, Miller JM, Rade JJ et al. Focal modification of electrical conduction in the heart by viral gene transfer. Nat Med 2000; 6: 1395–1398.
Tabata Y, Hijikata S, Muniruzzaman M, Ikada Y . Neovascularization effect of biodegradable gelatin microspheres incorporating basic fibroblast growth factor. J Biomater Sci Polym Ed 1999; 10: 79–94.
Kasahara H, Tanaka E, Fukuyama N, Sato E, Sakamoto H, Tabata Y et al. Biodegradable gelatin hydrogel potentiates the angiogenic effect of fibroblast growth factor 4 plasmid in rabbit hindlimb ischemia. J Am Coll Cardiol 2003; 41: 1056–1062.
Nagaya N, Kangawa K, Kanda M, Uematsu M, Horio T, Fukuyama N et al. Hybrid cell-gene therapy for pulmonary hypertension based on phagocytosing action of endothelial progenitor cells. Circulation 2003; 108: 889–895.
Tokunaga N, Nagaya N, Shirai M, Tanaka E, Ishibashi-Ueda H, Harada-Shiba M et al. Adrenomedullin gene transfer induces therapeutic angiogenesis in a rabbit model of chronic hind limb ischemia: benefits of a novel nonviral vector, gelatin. Circulation 2004; 109: 526–531.
Hosaka A, Koyama H, Kushibiki T, Tabata Y, Nishiyama N, Miyata T et al. Gelatin hydrogel microspheres enable pinpoint delivery of basic fibroblast growth factor for the development of functional collateral vessels. Circulation 2004; 110: 3322–3328.
Sakakibara Y, Tambara K, Sakaguchi G, Lu F, Yamamoto M, Nishimura K et al. Toward surgical angiogenesis using slow-released basic fibroblast growth factor. Eur J Cardiothorac Surg 2003; 24: 105–111; discussion 112.
Hayase M, Del Monte F, Kawase Y, Macneill BD, McGregor J, Yoneyama R et al. Catheter-based antegrade intracoronary viral gene delivery with coronary venous blockade. Am J Physiol Heart Circ Physiol 2005; 288: H2995–H3000.
Kornowski R, Fuchs S, Leon MB, Epstein SE . Delivery strategies to achieve therapeutic myocardial angiogenesis. Circulation 2000; 101: 454–458.
Grossman PM, Han Z, Palasis M, Barry JJ, Lederman RJ . Incomplete retention after direct myocardial injection. Catheter Cardiovasc Interv 2002; 55: 392–397.
Folkman J . Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1: 27–31.
Arras M, Mollnau H, Strasser R, Wenz R, Ito WD, Schaper J et al. The delivery of angiogenic factors to the heart by microsphere therapy. Nat Biotechnol 1998; 16: 159–162.
Boekstegers P, Kupatt C . Current concepts and applications of coronary venous retroinfusion. Basic Res Cardiol 2004; 99: 373–381.
Herity NA, Lo ST, Oei F, Lee DP, Ward MR, Filardo SD et al. Selective regional myocardial infiltration by the percutaneous coronary venous route: a novel technique for local drug delivery. Catheter Cardiovasc Interv 2000; 51: 358–363.
Fearon WF, Ikeno F, Bailey LR, Hiatt BL, Herity NA, Carter AJ et al. Evaluation of high-pressure retrograde coronary venous delivery of FGF-2 protein. Catheter Cardiovasc Interv 2004; 61: 422–428.
Nakazawa HK, Roberts DL, Klocke FJ . Quantitation of anterior descending vs circumflex venous drainage in the canine great cardiac vein and coronary sinus. Am J Physiol 1978; 234: H163–H166.
Hochberg MS, Roberts WC, Morrow AG, Austen WG . Selective arterialization of the coronary venous system. Encouraging long-term flow evaluation utilizing radioactive microspheres. J Thorac Cardiovasc Surg 1979; 77: 1–12.
Boekstegers P, Giehrl W, von Degenfeld G, Steinbeck G . Selective suction and pressure-regulated retroinfusion: an effective and safe approach to retrograde protection against myocardial ischemia in patients undergoing normal and high risk percutaneous transluminal coronary angioplasty. J Am Coll Cardiol 1998; 31: 1525–1533.
von Degenfeld G, Raake P, Kupatt C, Lebherz C, Hinkel R, Gildehaus FJ et al. Selective pressure-regulated retroinfusion of fibroblast growth factor-2 into the coronary vein enhances regional myocardial blood flow and function in pigs with chronic myocardial ischemia. J Am Coll Cardiol 2003; 42: 1120–1128.
Raake P, von Degenfeld G, Hinkel R, Vachenauer R, Sandner T, Beller S et al. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins: comparison with surgical and percutaneous intramyocardial gene delivery. J Am Coll Cardiol 2004; 44: 1124–1129.
Ortale JR, Gabriel EA, Iost C, Marquez CQ . The anatomy of the coronary sinus and its tributaries. Surg Radiol Anat 2001; 23: 15–21.
Thompson CA, Nasseri BA, Makower J, Houser S, McGarry M, Lamson T et al. Percutaneous transvenous cellular cardiomyoplasty. A novel nonsurgical approach for myocardial cell transplantation. J Am Coll Cardiol 2003; 41: 1964–1971.
Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol 2004; 44: 1690–1699.
Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 2004; 364: 141–148.
Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001; 410: 701–705.
Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA 2005; 102: 11474–11479.
Chien KR . Stem cells: lost in translation. Nature 2004; 428: 607–608.
Suzuki K, Murtuza B, Fukushima S, Smolenski RT, Varela-Carver A, Coppen SR et al. Targeted cell delivery into infarcted rat hearts by retrograde intracoronary infusion: distribution, dynamics, and influence on cardiac function. Circulation 2004; 110: II225–II230.
Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD . Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet 2004; 363: 783–784.
Blanton Jr JR, Grant AL, McFarland DC, Robinson JP, Bidwell CA . Isolation of two populations of myoblasts from porcine skeletal muscle. Muscle Nerve 1999; 22: 43–50.
De Grand AM, Frangioni JV . An operational near-infrared fluorescence imaging system prototype for large animal surgery. Technol Cancer Res Treat 2003; 2: 553–562.
Acknowledgements
We thank Research Institute for Production Development (Kyoto, Japan), Kirin Brewery Co. Ltd (Tokyo, Japan), Medtronic Vascular (CA, USA), and MID Co. Ltd (Fukuoka, Japan) for their financial support. This study was funded in part by a grant (R01-HL-78691) to Roger J Hajjar, MD from the National Heart Lung and Blood Institute.
We thank Jennifer McGregor, Catherine McMahon, and James Lough (Massachusetts General Hospital) for their technical help with animal care and handling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoshino, K., Kimura, T., De Grand, A. et al. Three catheter-based strategies for cardiac delivery of therapeutic gelatin microspheres. Gene Ther 13, 1320–1327 (2006). https://doi.org/10.1038/sj.gt.3302793
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302793
Keywords
This article is cited by
-
Targeted delivery of therapeutic agents to the heart
Nature Reviews Cardiology (2021)
-
Hydrogel microparticles for biomedical applications
Nature Reviews Materials (2019)
-
Magnetic targeting enhances retrograde cell retention in a rat model of myocardial infarction
Stem Cell Research & Therapy (2013)
-
Percutaneous Approaches for Efficient Cardiac Gene Delivery
Journal of Cardiovascular Translational Research (2013)
-
Percutaneous methods of vector delivery in preclinical models
Gene Therapy (2012)