Abstract
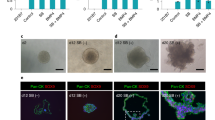
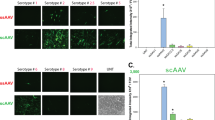
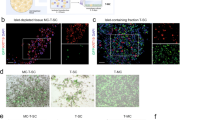
We previously demonstrated that recombinant adeno-associated virus vectors based on serotype 2 (rAAV2) can direct transgene expression in salivary gland cells in vitro and in vivo. However, it is not known how other rAAV serotypes perform when infused into salivary glands. The capsids of serotypes 4 and 5 are distinct from rAAV2 and from each other, suggesting that they may direct binding and entry into different cell types. In the present study, we investigated the tropisms, transduction efficiencies, and antibody response to AAV vectors based on AAV serotypes 2, 4, and 5. Administration of rAAV2β-galactosidase (βgal), rAAV4βgal, or rAAV5βgal to murine submandibular salivary glands by retrograde ductal instillation resulted in efficient transduction of salivary epithelial cells, with AAV4 and AAV5 producing 2.3 and 7.3 times more βgal activity compared with AAV2. Improved transduction with AAV5 was confirmed by QPCR of DNA extracted from glands and immunohistochemical staining for transgene expression. Like AAV2, AAV5 primarily transduced striated and intercalated ductal cells. AAV4 transduction was evident in striated, intercalated, and excretory ductal cells, as well as in convoluted granular tubules. In keeping with the encapsulated nature of the salivary gland, the majority of persistent viral genomes were found in the gland and not in other tissues. Neutralizing antibodies (NABs) found in the serum of virus-infused animals were serotype specific and there was no crossreactivity between serotypes. No NABs were detected in saliva but sialic acid conjugates present in saliva could neutralize AAV4 at low dilutions. Together our data suggest that because of differences in receptor binding and transduction pathways, other serotypes may have improved utility as gene transfer vectors in the salivary gland and these differences could be exploited in gene therapy applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Baum BJ, Goldsmith CM, Kok MR, Lodde BM, van Mello NM, Voutetakis A et al. Advances in vector-mediated gene transfer. Immunol Lett 2003; 90: 145–149.
Zufferey R, Aebischer P . Salivary glands and gene therapy: the mouth waters. Gene Therapy 2004; 22: 22.
Mastrangeli A, O'Connell B, Aladib W, Fox PC, Baum BJ, Crystal RG . Direct in vivo adenovirus-mediated gene transfer to salivary glands. Am J Physiol 1994; 266: G1146–G1155.
Zheng C, Baum BJ, Iadarola MJ, O'Connell BC . Genomic integration and gene expression by a modified adenoviral vector. Nat Biotechnol 2000; 18: 176–180.
Carter BJ . Adeno-associated virus and the development of adeno-associated virus vectors: a historical perspective. Mol Ther 2004; 10: 981–989.
Jooss K, Chirmule N . Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Therapy 2003; 10: 955–963.
Kok MR, Voutetakis A, Yamano S, Wang J, Cotrim A, Katano H et al. Immune responses following salivary gland administration of recombinant adeno-associated virus serotype 2 vectors. J Gene Med 2004; 28: 28.
Braddon VR, Chiorini JA, Wang S, Kotin RM, Baum BJ . Adenoassociated virus-mediated transfer of a functional water channel into salivary epithelial cells in vitro and in vivo. Hum Gene Ther 1998; 9: 2777–2785.
Voutetakis A, Kok MR, Zheng C, Bossis I, Wang J, Cotrim AP et al. Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics. Proc Natl Acad Sci USA 2004; 101: 3053–3058, Epub 2004 Feb 20.
Wang J, Voutetakis A, Zheng C, Baum BJ . Rapamycin control of exocrine protein levels in saliva after adenoviral vector-mediated gene transfer. Gene Therapy 2004; 11: 729–733.
Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ, Walsh CE . Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther 2000; 2: 619–623.
Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci USA 2000; 97: 3428–3432.
Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM . Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA 2002; 99: 11854–11859.
Zabner J, Seiler M, Walters R, Kotin RM, Fulgeras W, Davidson BL et al. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J Virol 2000; 74: 3852–3858.
Summerford C, Samulski RJ . Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol 1998; 72: 1438–1445.
Kashiwakura Y, Tamayose K, Iwabuchi K, Hirai Y, Shimada T, Matsumoto K et al. Hepatocyte growth factor receptor is a coreceptor for adeno-associated virus type 2 infection. J Virol 2005; 79: 609–614.
Mizukami H, Young NS, Brown KE . Adeno-associated virus type 2 binds to a 150-kilodalton cell membrane glycoprotein. Virology 1996; 217: 124–130.
Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A . Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med 1999; 5: 71–77.
Summerford C, Bartlett JS, Samulski RJ . AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med 1999; 5: 78–82.
Kaludov N, Brown KE, Walters RW, Zabner J, Chiorini JA . Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol 2001; 75: 6884–6893.
Di Pasquale G, Davidson BL, Stein CS, Martins I, Scudiero D, Monks A et al. Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med 2003; 9: 1306–1312, Epub 2003 Sep 14.
Yamano S, Huang LY, Ding C, Chiorini JA, Goldsmith CM, Wellner RB et al. Recombinant adeno-associated virus serotype 2 vectors mediate stable interleukin 10 secretion from salivary glands into the bloodstream. Hum Gene Ther 2002; 13: 287–298.
O'Connell BC, Zheng C, Jacobson-Kram D, Baum BJ . Distribution and toxicity resulting from adenoviral vector administration to a single salivary gland in adult rats. J Oral Pathol Med 2003; 32: 414–421.
Kok MR, Yamano S, Lodde BM, Wang J, Couwenhoven RI, Yakar S et al. Local adeno-associated virus-mediated interleukin 10 gene transfer has disease-modifying effects in a murine model of Sjogren's syndrome. Hum Gene Ther 2003; 14: 1605–1618.
Brockstedt DG, Podsakoff GM, Fong L, Kurtzman G, Mueller-Ruchholtz W, Engleman EG . Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin Immunol 1999; 92: 67–75.
Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J . Immune responses to adenovirus and adeno-associated virus in humans. Gene Therapy 1999; 6: 1574–1583.
Halbert CL, Miller AD . AAV-mediated gene transfer to mouse lungs. Methods Mol Biol 2004; 246: 201–212.
Walters RW, Pilewski JM, Chiorini JA, Zabner J . Secreted and transmembrane mucins inhibit gene transfer with AAV4 more efficiently than AAV5. J Biol Chem 2002; 277: 23709–23713.
Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol 2002; 76: 791–801.
Duan D, Yue Y, Yan Z, McCray Jr PB, Engelhardt JF . Polarity influences the efficiency of recombinant adenoassociated virus infection in differentiated airway epithelia. Hum Gene Ther 1998; 9: 2761–2776.
Chiorini J, Zabner J . Unpublished observation.
Slomiany BL, Murty VL, Piotrowski J, Slomiany A . Salivary mucins in oral mucosal defense. Gen Pharmacol 1996; 27: 761–771.
Kagami H, Atkinson JC, Michalek SM, Handelman B, Yu S, Baum BJ et al. Repetitive adenovirus administration to the parotid gland: role of immunological barriers and induction of oral tolerance. Hum Gene Ther 1998; 9: 305–313.
Halbert CL, Rutledge EA, Allen JM, Russell DW, Miller AD . Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J Virol 2000; 74: 1524–1532.
Kok MR, Baum BJ, Tak PP, Pillemer SR . Use of localised gene transfer to develop new treatment strategies for the salivary component of Sjogren's syndrome. Ann Rheum Dis 2003; 62: 1038–1046.
Baum BJ, Voutetakis A, Wang J . Salivary glands: novel target sites for gene therapeutics. Trends Mol Med 2004; 10: 585–590.
Voutetakis A, Bossis I, Kok MR, Zhang W, Wang J, Cotrim AP et al. Salivary glands as a potential gene transfer target for gene therapeutics of some monogenetic endocrine disorders. J Endocrinol 2005; 185: 363–372.
Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol 2004; 78: 6381–6388.
Mori S, Wang L, Takeuchi T, Kanda T . Two novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid protein. Virology 2004; 330: 375–383.
Walters RW, Yi SM, Keshavjee S, Brown KE, Welsh MJ, Chiorini J et al. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem 2001; 276: 20610–20616.
Wang S, Baum BJ, Yamano S, Mankani MH, Sun D, Jonsson M et al. Adenoviral-mediated gene transfer to mouse salivary glands. J Dent Res 2000; 79: 701–708.
Acknowledgements
We acknowledge the contributions of Beverly Handelman for technical, and Roberta Knox and Karen Knight for administrative, assistance. We thank Antonis Voutetakis for critical review and helpful discussion of this manuscript and Bob Redman for assistance with cell histology. MR Kok was supported by the Dutch Arthritis Foundation nr 02-01-302.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katano, H., Kok, M., Cotrim, A. et al. Enhanced transduction of mouse salivary glands with AAV5-based vectors. Gene Ther 13, 594–601 (2006). https://doi.org/10.1038/sj.gt.3302691
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302691
Keywords
This article is cited by
-
Neutralizing antibodies against adeno-associated viruses in Sjögren’s patients: implications for gene therapy
Gene Therapy (2017)
-
Local delivery of AAV2-CTLA4IgG decreases sialadenitis and improves gland function in the C57BL/6.NOD-Aec1Aec2 mouse model of Sjögren's syndrome
Arthritis Research & Therapy (2012)
-
AAV2-mediated transfer of the human aquaporin-1 cDNA restores fluid secretion from irradiated miniature pig parotid glands
Gene Therapy (2011)
-
Adenoviral delivery of Tousled kinase for the protection of salivary glands against ionizing radiation damage
Gene Therapy (2011)
-
AAV5-mediated gene transfer to the parotid glands of non-human primates
Gene Therapy (2010)