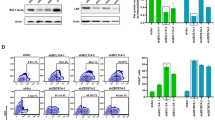
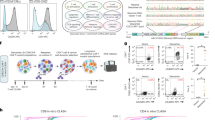
Abstract
RNA interference (RNAi) has recently been used for sequence-specific gene silencing of disease-related genes including oncogenes in hematopoietic cells. To characterize its potential therapeutic value, we analyzed different modes to activate RNAi as well as some pharmacokinetic aspects of gene silencing in bcr-abl+ cells. Using lentiviral gene transfer of transcription cassettes for anti-bcr-abl shRNAs and red fluorescence protein (RFP) as a quantitative reporter, we demonstrate that stable but not transient RNAi can efficiently deplete bcr-abl+ K562 and murine TonB cells from suspension cultures. Importantly, depletion of bcr-abl+ cells depends on the dose of lentivirus used for transduction and correlates with the RFP-expression level of transduced target cells: RFP-high K562 cells are eradicated, whereas RFP-low or -intermediate cells may recover after prolonged cell culture. Interestingly, these cells still show reduced bcr-abl mRNA levels, aberrant proliferation kinetics, and enhanced sensitivity to the Bcr-Abl-kinase inhibitor STI571. Quantitative PCR from genomic DNA suggests that more than three lentiviral integrations are required for effective depletion of K562 cells. Finally, we demonstrate that lentivirus-mediated anti-bcr-abl RNAi can inhibit colony formation of primary CD34+ cells from chronic myeloid leukemia patients. These data demonstrate dose-dependent gene silencing by lentivirus-mediated RNAi in bcr-abl+ cells and suggest that stable RNAi may indeed be therapeutically useful in primary hematopoietic cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Elbashir S et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001; 411: 494–498.
Caplen NJ et al. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc Natl Acad Sci USA 2001; 98: 9742–9747.
Zamore PD . RNA interference: listening to the sound of silence. Nat Struct Biol 2001; 8: 746–750.
Paddison PJ et al. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev 2002; 16: 948–958.
Brummelkamp TR, Bernards R, Agami R . A system for stable expression of short interfering RNAs in mammalian cells. Science 2002; 296: 550–553.
Hannon GJ . RNA interference. Nature 2002; 418: 244–251.
Novina CD et al. siRNA-directed inhibition of HIV-1 infection. Nat Med 2002; 8: 681–686.
Lee NS et al. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat Biotechnol 2002; 20: 500–505.
Kapadia SB, Brideau-Andersen A, Chisari FV . Interference of hepatitis C virus RNA replication by short interfering RNAs. Proc Natl Acad Sci USA 2003; 100: 2014–2018.
Wilson JA et al. RNA interference blocks gene expression and RNA synthesis from hepatitis C replicons propagated in human liver cells. Proc Natl Acad Sci USA 2003; 100: 2783–2788.
McCaffrey AP et al. Inhibition of hepatitis B virus in mice by RNA interference. Nat Biotechnol 2003; 21: 639–644.
Song E et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med 2003; 9: 347–351.
Park WS, Hayafune M, Miyano-Kurosaki N, Takaku H . Specific HIV-1 env gene silencing by small interfering RNAs in human peripheral blood mononuclear cells. Gene Therapy 2003; 10: 2046–2050.
Wilda M, Fuchs U, Wossmann W, Borkhardt A . Killing of leukemic cells with a BCR/ABL fusion gene by RNA interference (RNAi). Oncogene 2002; 21: 5716–5724.
Scherr M et al. Specific inhibition of bcr-abl gene expression by small interfering RNA. Blood 2003; 101: 1566–1569.
Wohlbold L et al. Inhibition of bcr-abl gene expression by small interfering RNA sensitizes for imatinib mesylate (STI571). Blood 2003; 102: 2236–2239.
Heidenreich O et al. AML1/MTG8 oncogene suppression by small interfering RNAs supports myeloid differentiation of t(8;21)-positive leukemic cells. Blood 2003; 101: 3157–3163.
Scherr M, Steinmann D, Eder M . RNA interference (RNAi) in hematology. Ann Hematol 2004; 83: 1–8.
Barton GM, Medzhitov R . Retroviral delivery of small interfering RNA into primary cells. Proc Natl Acad Sci USA 2002; 99: 14943–14945.
Devroe E, Silver PA . Retrovirus-delivered siRNA. BMC Biotechnol 2002; 2: 7–15.
Abbas-Terki T et al. Lentiviral-mediated RNA interference. Hum Gene Ther 2002; 13: 2197–2201.
Rubinson DA et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet 2003; 33: 401–406.
Kunath T et al. Transgenic RNA interference in ES cell-derived embryos recapitulates a genetic null phenotype. Nat Biotechnol 2003; 21: 559–561.
Xia H, Mao Q, Paulson HL, Davidson BL . siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol 2002; 20: 1006–1010.
Stewart SA et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 2003; 9: 493–501.
Carmell MA et al. Transmission of RNAi in mice. Nat Struct Biol 2003; 10: 91–92.
Scherr M, Battmer K, Ganser A, Eder M . Modulation of gene expression by lentiviral-mediated delivery of small interfering RNA. Cell Cycle 2003; 2: 251–257.
Scherr M et al. Inhibition of GM-CSF receptor function by stable RNA interference in a NOD/SCID mouse hematopoietic stem cell transplantation model. Oligonucleotides 2003; 13: 353–363.
Li MJ et al. Specific killing of Ph+ chronic myeloid leukemia cells by a lentiviral vector-delivered anti-bcr/abl small hairpin RNA. Oligonucleotides 2003; 13: 401–409.
Bridge AJ et al. Induction of an interferon response by RNAi vectors in mammalian cells. Nat Genet 2003; 34: 263–264.
Schwarz DS et al. Asymmetry in the assembly of the RNAi enzyme complex. Cell 2003; 115: 199–208.
Khvorova A, Reynolds A, Jayasena SD . Functional siRNAs and miRNAs exhibit strand bias. Cell 2003; 115: 209–216.
Reynolds A et al. Rational siRNA design for RNA interference. Nat Biotechnol 2004; 22: 326–330.
Lee YS et al. Distinct roles for Drosophila dicer-1 and dicer-2 in the siRNA/miRNA silencing pathway. Cell 2004; 117: 69–81.
Pham JW et al. A dicer-2-dependent 80S complex cleaves targeted mRNAs during RNAi in Drosophila. Cell 2004; 117: 83–94.
Baum C et al. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood 2003; 101: 2099–2114.
Klucher KM, Lopez DV, Daley GQ . Secondary mutation maintains the transformed state in BaF3 cells with inducible BCR/ABL expression. Blood 1998; 91: 3927–3934.
Scherr M et al. Lentiviral gene transfer into peripheral blood-derived CD34+ NOD/SCID-repopulating cells. Blood 2002; 99: 709–712.
Eder M et al. Monitoring of BCR-ABL expression using real-time RT-PCR in CML after bone marrow or peripheral blood stem cell transplantation. Leukemia 1999; 13: 1383–1389.
Bieche I et al. Novel approach to quantitative polymerase chain reaction using real-time detection: application to the detection of gene amplification in breast cancer. Int J Cancer 1998; 78: 661–666.
Schiedlmeier B et al. Quantitative assessment of retroviral transfer of the human multidrug resistance 1 gene to human mobilized peripheral blood progenitor cells engrafted in nonobese diabetic/severe combined immunodeficient mice. Blood 2000; 95: 1237–1248.
Maniatis T, Fritsch EF, Sambrook J . Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, 1989.
Acknowledgements
This work was supported in part by grants of the HW and J Hector-Stiftung, the Wilhelm Sander-Stiftung, and the ‘Deutsche Forschungsgemeinschaft’ (SFB 566). We thank George Daley (MIT, Cambridge) for providing us with the TonB cell line used in this study.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Scherr, M., Battmer, K., Schultheis, B. et al. Stable RNA interference (RNAi) as an option for anti-bcr-abl therapy. Gene Ther 12, 12–21 (2005). https://doi.org/10.1038/sj.gt.3302328
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302328
Keywords
This article is cited by
-
Inhibition of multi-drug resistance of ovarian carcinoma by small interfering RNA targeting to MRP2 gene
Archives of Gynecology and Obstetrics (2009)
-
Inhibition of Abl tyrosine kinase enhances nerve growth factor-mediated signaling in Bcr–Abl transformed cells via the alteration of signaling complex and the receptor turnover
Oncogene (2008)
-
RNAi-mediated silencing of p190Bcr-Abl inactivates Stat5 and cooperates with imatinib mesylate and 17-allylamino-17-demetoxygeldanamycin in selective killing of p190Bcr-Abl-expressing leukemia cells
Leukemia (2008)
-
Transient or long-term silencing of BCR-ABL alone induces cell cycle and proliferation arrest, apoptosis and differentiation
Leukemia (2006)
-
Effects of siRNAs in combination with Gleevec on K-562 cell proliferation and Bcr-Abl expression
Journal of Biomedical Science (2006)