ABSTRACT
Trail, a tumor necrosis factor-related apoptosis-inducing ligand, is a novel potent endogenous activator of the cell death pathway through the activation of cell surface death receptors Trail-R1 and Trail-R2. Its role, like FasL in activation-induced cell death (AICD), has been demonstrated in immune system. However the mechanism of Trail induced apoptosis remains unclear. In this report, the recombinant Trail protein was expressed and purified. The apoptosis-inducing activity and the regulation mechanism of recombinant Trail on Jurkat T cells were explored in vitro. Trypan blue exclusion assay demonstrated that the recombinant Trail protein actively killed Jurkat T cells in a dose-dependent manner. Trail-induced apoptosis in Jurkat T cells were remarkably reduced by Bcl-2 over expression in Bcl-2 gene transfected cells. Treatment with PMA (phorbol 12-myristate 13-acetate), a PKC activator, suppressed Trail-induced apoptosis in Jurkat T cells. The inhibition of apoptosis by PMA was abolished by pretreatment with Bis, a PKC inhibitor. Taken together, it was suggested that Bcl-2 over-expression and PMA activated PKC actively down-regulated the Trail-mediated apoptosis in Jurkat T cell.
Similar content being viewed by others
INTRODUCTION
Trail1 (TNF-related apoptosis inducing ligand) is a recently described member of the TNF family. Like other members of the TNF ligand family, Trail could induce apoptosis of neoplastically transformed cells by activating cell surface death receptors Trail-R1 and Trail-R22.
Trail has been demonstrated to play an important role in homeostasis of immune system including eradication of the old lymphocyte3, activation-induced T cell death4, regulation of T cell expansion by B cells, and T cell-mediated cytotoxicity etc. T and B lymphocytes express surface Trail that participates in activation induced cell death (AICD). However, the regulation of Trail protein in immune cells has not been fully determined.
It is widely accepted that apoptosis is controlled by several genetic programs. In the past decade, studies have demonstrated that there are two major cell intrinsic pathways for regulating apoptosis: one is receptor-mediated pathway, for example Fas-FasL pathway, the other is mitochondria-mediated pathway, such as Bcl-2 family genes. The two pathways were directly linked by Bid, a proapoptotic member of Bcl-2 family5. Bcl-2 inhibits apoptosis induced by many signals such as ultra-violet radiation, anti-tumor drugs and deprivation of growth factor. We also find that Bcl-2 inhibits FasL-induced apoptosis in hepatoma BEL-74046. The effect of Bcl-2 on Trail-induced apoptosis remains controversial, for example Bcl-2 can block Trail-induced apoptosis in glioma LN-229 cells7 but not in myeloma ARP-1 cells8. It seems that the Trail shows different effects on different cell types.Protein kinase C (PKC), a serine/threonine kinase that plays an essential role in the phosphorylation of proteins involved in signal transduction pathways, can inhibit FasL induced apoptosis in Jurkat T cells9, but whether it regulates the Trail-induced apoptosis remains to be investigated.
The aim of this study is to investigate the regulation of Trail-induced apoptosis and examine whether the activation of PKC and over-expression of Bcl-2 affect Trail-induced apoptosis in Jurkat T cells. In the present work, we cloned and expressed recombinant Trail protein and confirmed that the recombinant Trail protein could induce apoptosis in Jurkat T cell. PMA inhibited Trail-induced apoptosis in Jurkat T cells, while the Bis, an inhibitor of PKC, abolished the inhibitory effect of PMA on the Trail-induced apoptosis. In addition, we also examined and found no effect of other immunoactivator including LPS, IL-2, PHA on Trail-induced apoptosis. Over-expression of Bcl-2 constructed by transfecting the Bcl-2 gene into Jurkat T cells could suppress Trail-induced apoptosis in Jurkat T cell. The results suggested that Bcl-2 over-expression and PKC activation inhibited Trail-induced apoptosis in Jurkat T cell.
MATERIALS AND METHODS
Chemicals
Phorbol 12-myristate 13-acetate (PMA), Bis (bisindolylmaleimide I, HCl), PHA, IL-2, Diazobenzidine (DAB), G418 were purchased from Sigma Chemical Co. Anti-Bcl-2 antibody and HRP-conjugated second antibody were purchased from Santa Cruz Biotechnology Inc.
Cell culture
Jurkat T cells (human T lym phoma, CAS Cell Bank) were routinely cultured in RPMI 1640 medium (GibcoBRL) supplemented with 10% newborn bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were maintained at 37°C in a humidified atmosphere with 5% CO2. Cell viability was assessed by trypan blue exclusion. Expression and purification of Trail protem.
Expression and purification of Trail protein
Prokaryotic expressing plasmid pT7-6His-Trail was transformed into E.coli. BL21(DE3) and induced to express 6His-Trail fusion protein at 37°C under 0.1 mM IPTG induction for 4 h. The cell culture was harvested and washed with sonication buffer for 3 times. Then the cells were resuspended in sonication buffer and incubated on ice for 30 min after adding 1 mg/ml lysozyme and PMSF. The cells were sonicated on ice and centrifuged at 12000×g for 30 min at 4 °C. The supernatant was collected and mixed with pre-equilibrated Ni+-NTA resin in sonication buffer for binding for 1 h at 4 °C. After washing the mixture for 3 times, the protein was eluted with elution buffer (0.3 M imidazole in sonication buffer). The eluate was collected, and imidazole was removed by dialysis in PBS. The precipitate was also collected and washed with washing buffer for three times. The purity of purified recombinant protein was inspected by PAGE electrophoresis and the concentration of purified recombinant protein was determined by Bradford method. Finally, the purified recombinant protein was stored at −20°C in 100 μl fractions.
Transient transfection of Jurkat T cells with BCL-2 gene (using electroporation method)
The apparatus used in the experiment was Gene PulserTM (BIORAD). Jurkat T cells growing to exponential stage were pooled, rinsed twice with D-Hanks saline solution and suspended in D-Hanks at 0.5–1.0×107 cells/ml. Eukaryotic expressing Plasmid pcDNA3-Bcl-2 (Bcl-2 cDNA was inserted into vector pcDNA3 at EcoR I site) was added to cells culture at a final concentration of 30 μg/ml and incubated on ice for 10 min. Then cells were electroporated at 500V, 25μF and 100 Ω and incubated on ice for another 10 min. Appropriate amount of cells were inoculated into RMPI1640 and developed in 37°C, 5%CO2. The transfected Jurkat T cells were selected with 800 μg/ml G418 for 2 w.
Western blot analysis
The Bcl-2 protein expression was determined by western blot analysis. In brief, 10μg of total protein extracted from Jurkat T cells or transfected cells were separated by SDS-PAGE, transferred onto nitrocellulose membrane and probed with mouse anti-human Bcl-2 monoclonal antibody. Membrane was subsequently incubated with HRP-conjugated second antibody and finally developed with diaminobenzidine and H2O2.
Apoptosis assay
The induction of apoptosis of Jurkat T cells by treatment with purified recombinant Trail protein in different doses and times was assessed by examination of cellular morphological changes under light microscopy and nuclear division stained with propidium iodide (PI) by fluorescent microscope. In brief, the harvested cells were washed three times with D-Hanks and fixed in sodium citrate at 4°C for 30 min. 500 μl staining solution (PI 50 μg/ml, RNase 10 μg/ml) was added to 106 cells and incubated at 4°C for 15 min in dark before the microscope examination.
Effect of PKC activity on trail-induced apoptosis
The Jurkat T cells were pretreated with PMA (10 ng/ml), PHA (50 μg/ml), IL-2(10U/ml), LPS (100 ng/ml) for 12 h, followed by 100 ng/ml Trail protein treatment for additional 12 h. Cell viability was assessed by trypan blue exclusion. In PKC activity assay, before treatment with PMA the Jurkat T cells were incubated with Bis (0.15 μM) for 4 h. The apoptotic experiments were repeated at least three times.
RESULTS
Expression and purification of recombinant Trail protein
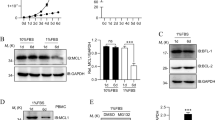
The Trail gene was cloned by RT-PCR and the sequence was confirmed by sequencing (data not shown). The recombinant Trail protein was expressed in E.coli. BL21 (DE3) and purified with Ni+-NTA agarose column. The purified 21kDa recombinant Trail protein (shown in Fig 1) was used in the following experiments to detect its effect on apoptosis induction in Jurkat T cells.
Recombinant Trail protein induces apoptosis in Jurkat T cells
In order to investigate the biological activity of recombinant Trail protein on Jurkat T cells, various dosages of purified protein were added to treat the Jurkat T cells. In culture, the Jurkat T cells grew and proliferated into aggregated groups (Fig 2A). After 4 h treatment with 100 ng/ml Trail protein, the cells began to appear the characteristics of apoptosis showing cell disaggregation, and cellular shrinkage (Fig 2B). Furthermore the condensation of chromatin and breakdown of the nucleus into several smalls pieces were also seen (Fig 2C). The occurrence of apoptosis was quantitated by Typan Blue assay. As shown in Tab 1, the Jurkat T cells were very sensitive to recombinant Trail protein treatment, resulting in an induction of apoptosis in a dose-dependent manner.
Over-expression of Bcl-2 delays Trail induced-apoptosis in Jurkat T cells
Bcl-2 as an anti-apoptosis gene was initially isolated from a follicular lymphoma and has been shown to enhance the survival of a variety of cell types exposed to diverse cell death-inducing stimuli. To investigate the effect of Bcl-2 protein on Trail-induced apoptosis in Jurkat T cells, a reconstructed eukaryotic expressing vector pcDNA3-Bcl-2 was transfected into Jurkat T cells by electroporation. The cells were selected for 2 w with 800mg/ml G418. The results showed that the Jurkat T cells transfected with Bcl-2 expressing vector expressed 26 kDa Bcl-2 protein as showed in Western blot of Fig 3A in comparison with control parent Jurkat T cells and pcDNA3 plasmid transfected cells.
Analysis of the effect of Bcl-2 over-expression on Trail-induced apoptosis in Jurkat T cells (A) Western blot analysis of Bcl-2 protein expression in Jurkat T cell. 1. Parent Jurkat T cell. 2. Jurkat T cells transfected with empty plasmid pcDNA3. 3. Jurkat T cells transfected with expression plasmid pcDNA3-Bcl-2. (B) Bcl-2 over expression block Trail induced apoptosis in Jurkat T cells. 1. Parent Jurkat T cell. 2. Jurkat T cell transfected with pcDNA3. 3. Jurkat T cell transfected with expression plasmid pcDNA3-Bcl-2. The data were the mean±SD of 3 independent experiments
Bcl-2 over-expressing Jurkat T cells, parent Jurkat T cell and pcDNA3 transfected Jurkat T cells were treated with 100 ng/ml purified Trail protein for 12 h. The results showed that the Trail protein induced 83.2% of parent Jurkat T cells to undergo apoptosis, while the Bcl-2 protein over-expressed Jurkat T cells showed remarkable resistance to Trail-induced apoptosis, resulting in a reduction of apoptosis rate from 83.2% to 32.6% of the cells. There was no significant effect of pcDNA3 transfection on cells apoptosis induced by Trail (Fig 3B). These results indicated that over-expression of Bcl-2 obviously suppressed the apoptosis induced by Trail in Jurkat T cells.
PMA inhibits apoptosis induced by Trail in Jurkat T cell through activation of PKC
PMA is an effective activator of PKC, which participates in regulating apoptosis induced by many stimuli, for example FasL in Jurkat T cells. To investigate whether PMA had inhibitory effect on Trail-induced apoptosis, Jurkat T cells were pretreated with PMA (10 ng/ml) for 12 h followed by incubation with 100 ng/ml recombinant Trail for additional 12 h. As shown in Fig 4, PMA significantly protected Jurkat T cells from the Trail-induced apoptosis, reducing the apoptotic cells rate to 11.7%, in comparison with 82.4% apoptosis of control Jurkat T cells during incubation with same amount of Trail. In order to investigate whether the activation of PKC during the PMA pretreatment was the cause of protection in Jurkat T cells, Bis (0.15 μM), an inhibitor of PKC, was added for 4 h before the treatment of PMA (10 ng/ml) to inhibit the activity of PKC. The results in Fig 5 indicated that Bis pretreatment obviously abolished the apoptosis-inhibiting effect of PMA, while Bis alone had no effect on Trail-treated Jurkat T cells. These results implied that the activation of PKC pathway might be responsible for the inhibition of apoptosis by PMA in the Trail-treated Jurkat T cells.
In addition, we examined the effect of other immunoactivator such as LPS, PHA and IL-2 on Trail-induced apoptosis. Jurkat T cells were pretreated with PHA (50 μg/ml), IL-2 (10 U/ml) and LPS (100 ng/ml) for 12 h followed by incubation with 100 ng/ml recombinant Trail for additional 12 h. As shown in Fig 6, LPS, PHA and IL-2 pretreatment, compared with contral, had little effect on Trail induced apoptosis in Jurkat T cells. The rates of Trail induced apoptosis in LPS, PHA and IL-2 pretreated Jurkat T cells were 85.2%, 79.6% and 80.4% respectively, no significant difference from 82.4% in Trail-treated Jurkat T cells.
DISCUSSION
Lymphocyte death by apoptosis is an essential mechanism for maintaining homeostasis of immune system. The expression of cell surface receptors such as CD40, CD20, CD2, Fas, TNFR and Trail-Rs seems to be required in determining whether lymphocytes survive or undergo apoptosis10. Like FasL, Trail triggers apoptotic death by activating the cell surface death receptor Trail-R1 and Trail-R2 2. It is further comfirmed that Trail receptors Trail-R3 and Trail-R4 with truncated cytoplasmic region can protect the target cells from Trail-induced apoptosis.
Bcl-2 is a anti-apoptosis gene that can inhibit apoptosis induced by many stimuli, for example, irradiation, TNF-α, FasL and deprivation of growth factor. The effect of Bcl-2 on Trail-induced apoptosis are controversial 7,8 mainly depending on different cell types. In order to investigate the effect of Bcl-2 on Trail-induced apoptosis in Jurkat T cells, we inspected the endogenous Bcl-2 expression and found the expression level of Bcl-2 was low (Fig 2 lane 2). We did find that Bcl-2 over-expression could suppress Trail-induced apoptosis in Jurkat T cells. These results suggested that the effect of Bcl-2 on Trail-induced apoptosis might depend on cell types and their properties. There are two subclasses of Bcl-2 family functioning as either anti-apoptosis regulators including Bcl-2, Bcl-XL pro-apoptotic proteins, for example, Bid, Bax etc. Bid existed in the cytosolic fraction of living cells as an inactive precursor. It was reported that Bid protein was activated by cleavage of precursor via activation of caspase 8 triggered by Trail in human B lymphoma BJAB cells and FasL in Jurkat T cells11,12. It has been demonstrated that FADD and caspase 8 participate in the apoptotic signal pathway 13. Our results suggested that the Trail induced cell death in Jurkat T cells might depend on the Bid activation resulted from Trail treatment caused caspase 8 activities, because Bcl-2 over-expression protected the Jurkat T cells from Trail induced apoptosis by competitively heterodimerizing with Bid protein.
Bcl-2 gene expression not only suppresses Trail-induced apoptosis in Jurkat T cell but also inhibits FasL-induced apoptosis in hepatoma cells6. Previous reports have revealed that activation of PKC could inhibit FasL-induced apoptosis in Jurkat T cells9. It is interesting to see whether PKC might also participate in the regulation Trail-induced apoptosis. In this study, we found that PMA, a PKC activator, could inhibit Trail-induced apoptosis in Jurkat T cell and this inhibitory effect could be abolished by Bis, an inhibitor of PKC. Our results demonstrated that PMA inhibited Trail-induced apoptosis by activating PKC.
It was reported that the adjustment of Fas-mediated apoptosis by activation of PKC occurred at a site upstream of caspase-8 and caspase-39. It suggested that PKC may regulate the activities of these cysteine proteases through unknown molecules existing upstream of caspase 8 which play a center role in Trail-induced apoptosis, because caspase-8 inhibitor can inhibit Trail- induced apoptosis14. When PKC was activated it could inhibit many stimulus induced apoptosis in cells, such as UV in U937 cells15, TNF-α and FasL in human lymphoma cells16, 17. Han 18 demonstrated that PKC took part in regulating the release of cytochrome C and Ro-31-8220 (an inhibitor of PKC) could induce apoptosis in HL-60 cells by the process of mitochondrial cytochrome C efflux and the activation of caspase-3. In this study, we found that PKC activation drived by PMA treatment on Jurkat T cells could inhibit Trail-induced apoptosis. The inhibitory effect of PMA might be due to expression of an unknown proteins existing upstream of caspase-8 that could inhibit the activation of caspase-8 or caspase-3.
Our results suggested that the level of Bcl-2 protein and activity of PKC affected the anti-tumor activity of Trail. Decrease of Bcl-2 expression and inhibition of PKC activity might enhance the anti-tumor activity of Trail. It is helpful for design of a new tumor therapy stratgy.
References
Pitti RM, Marsters SA, Ruppert S et al. Induction of apoptosis by Apo-2 Ligand a new member of the Tumor necrosis factor cytokine family. J Biol Chem 1996; 271(22):12687–90.
Yakenari T, Shiraki K, Sugimoto K et al. Chemotherapeutic agents augment Trail-induced apoptosis in human hepatocellular carcinoma cell lines. Hepatology. 2000; 32(3):482–9.
Okada H, Kobune F, Sato TA, et al. Extensive lymphopenia due to apoptosis of uninfected lymphocytes in acute measles patients. Arch Virol 2000; 145:5905–20.
Martinez Lorenzo MJ, Alava MA, Gamen S, et al. Involvement of APO2 ligand/TRAIL in activation-induced death of Jurkat and human peripheral blood T cells. Eur J Immunol. 1998; Sep 28:9 2714–25.
Luo X, Budihardjo I, Zuo H, et al. Bid, a Bcl-2 interacting peotein, mediates cytochrome C release from mitochondria in response to activation of cell surface death receptors. Cell 1998; 94:481–90.
Chang YC, Xu YH . Expression of Bcl-2 inhibited Fas-mediated apoptosis in human hepatocellular carcinoma BEL-7404 cells. Cell Research 2000; 10:233–42.
Rieger J, Naumann U, Glaser T et al. APO2 ligand: a novel lethal weapon against malignant glioma? FEBS Lett 1998; 427(1):124–8.
Gazitt Y, Shaughnessy P and Montgomery W . Apoptosis-induced by TRAIL AND TNF-alpha in human multiple myeloma cells is not blocked by BCL-2. Cytokine 1999; 11(12):1010–9.
Gomez-Angelats M, Bortner CD and Cidlowski JA . Protein kinase C (PKC) inhibits fas receptor-induced apoptosis through modulation of the loss of K+and cell shrinkage. A role for PKC upstream of caspases. J Biol Chem 2000; 275(26):19609–19.
Hoskin DW . Trail: a newly described effector mechanism of cytotoxic lymphocytes. Mod Asp Immunobiol 1(4):136–9.
Yamada H, Tada-Oikawa S, Uchida A and Kawanishi S . TRAIL causes cleavage of bid by caspase-8 and loss of mitochondrial membrane potential resulting in apoptosis in BJAB cells. Biochem Biophys Res Commun 1999; 265(1):130–3.
Cuvillier O, Edsall L and Spiegel S . Involvement of sphingosine in mitochondria-dependant fas-induced apoptosis of type Jurkat T cells. J Biol Chem 2000; 275(21):15691–700.
Sprick MR, Weigand MA, Rieser E et al. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 2000; 12(6):599–609.
Griffith TS, Anderson RD, Davidson BL, et al. Adenoviral-mediated transfer of the TNF-related apoptosis-inducing ligand/Apo-2 ligand gene induces tumor cell apoptosis. J Immunol 2000; 165(5):2886–94.
Yoko SK, Kyoji Ikeda and Makoto Nakanishi . Phorbol ester inhibits DNA damage-induced apoptosis in U937 cells through activation of protein kinase C. Life Science 1999; 65(21):2251–8.
Obeid LM, Linardic CM, Karolak LA et al. Programmed cell death induced by ceramide. Science 1993; 259:1769–71.
Takano Y and Okudaira M . Apoptosis induced by microtubule disrupting drugs in cultured human lymphoma cell. inhibitory effects of phorbol ester and zinc sulphate. Pathol. Res Pract 1993; 189(2):197–203.
Han Z, Pantazis P, Lange TS et al. The staurosporine analog, Ro-31-8220, inducesapoptosis independently of its ability to inhibit protein kinase C. Cell Death Differ. 2000; 7(6):521–30.
Acknowledgements
This work was supported by Major State Basic Research (973) Program of China, (G1999053905).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
GUO, B., XU, Y. Bcl-2 over-expression and activation of protein kinase C suppress the Trail-induced apoptosis in Jurkat T cells. Cell Res 11, 101–106 (2001). https://doi.org/10.1038/sj.cr.7290074
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290074
Keywords
This article is cited by
-
Role of Bax/Bcl-2 family members in green tea polyphenol induced necroptosis of p53-deficient Hep3B cells
Tumor Biology (2014)
-
Homoharringtonine acts synergistically with SG235-TRAIL, a conditionally replicating adenovirus, in human leukemia cell lines
Acta Pharmacologica Sinica (2009)
-
Induction of mitochondrion-mediated apoptosis of CHO cells by tripchloro- lide
Cell Research (2003)
-
Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression
Cell Research (2003)
-
The associated regulators and signal pathway in rIL-16/CD4 mediated growth regulation in Jurkat cells
Cell Research (2002)