Abstract
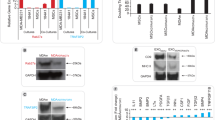
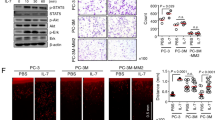
The EphA2 receptor tyrosine kinase is frequently overexpressed in invasive breast cancer cells. Moreover, these malignant cells have unstable cell–cell contacts, which preclude EphA2 from interacting with its ligand, EphrinA1, which is anchored to the membrane of adjacent cells. This defect is important because ligand binding causes EphA2 to transmit signals that negatively regulate tumor cell growth and survival, whereas the absence of ligand binding favors these same behaviors. In our present study, human adenoviral type 5 (HAd) vectors were engineered to express secreted-forms of EphrinA1. These vectors were used to infect MDA-MB-231 human breast cancer cells, or MCF-10A human breast epithelial cells providing matched controls. Infection with HAd-EphrinA1-Fc (HAd vector expressing extracellular domain of human EphrinA1 attached to Fc portion of human IgG1 heavy chain) caused increased EphA2 activation and turnover and consequently decreased tumor cell viability in soft agar assays. Consistent with this observation, infection of MDA-MB-231 cells with HAd-EphrinA1-Fc prevented tumor formation in xenograft models. Furthermore, therapeutic modeling via intratumoral inoculation revealed that HAd-EphrinA1-Fc significantly inhibited subsequent tumor growth as compared to matched controls. These results suggest that targeting of EphA2 with adenoviral vectors may have therapeutic value.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dickson RB, Lippman ME . Growth factors in breast cancer. Endocr Rev. 1995;16:559–589.
Zelinski DP, Zantek ND, Stewart JC, et al. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306.
Rosenberg IM, Goke M, Kanai M, et al. Epithelial cell kinase-B61: an autocrine loop modulating intestinal epithelial migration and barrier function. Am J Physiol. 1997;273:G824–G832.
Easty DJ, Guthrie BA, Maung K, et al. Protein B61 as a new growth factor: expression of B61 and up-regulation of its receptor epithelial cell kinase during melanoma progression. Cancer Res. 1995;55:2528–2532.
Andres AC, Zuercher G, Djonov V, et al. Protein tyrosine kinase expression during the estrous cycle and carcinogenesis of the mammary gland. Int J Cancer. 1995;63:288–296.
Walker-Daniels J, Coffman K, Azimi M, et al. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. 1999;41:275–280.
Dohn M, Jiang J, Chen X . Receptor tyrosine kinase EphA2 is regulated by p53-family proteins and induces apoptosis. Oncogene. 2001;20:6503–6515.
Miao H, Wei BR, Peehl DM, et al. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 2001;3:527–530.
Coffman KT, Hu M, Carles-Kinch K, et al. Differential EphA2 epitope display on normal versus malignant cells. Cancer Res. 2003;63:7907–7912.
Kinch MS, Carles-Kinch K . Overexpression and functional alterations of the EphA2 tyrosine kinase in cancer. Clin Exp Metast. 2003;20:59–68.
Bartley TD, Hunt RW, Welcher AA, et al. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature. 1994;368:558–560.
Zisch AH, Pazzagli C, Freeman AL, et al. Replacing two conserved tyrosines of the EphB2 receptor with glutamic acid prevents binding of SH2 domains without abrogating kinase activity and biological responses. Oncogene. 2000;19:177–187.
Kullander K, Mather NK, Diella F, et al. Kinase-dependent and kinase-independent functions of EphA4 receptors in major axon tract formation in vivo. Neuron. 2001;29:73–84.
Walker-Daniels J, Hess AR, Hendrix MJC, et al. Differential regulation of EphA2 in normal and malignant cells [Review]. Am J Pathol. 2003;162:1037–1042.
Koolpe M, Dail M, Pasquale EB . An ephrin mimetic peptide that selectively targets the EphA2 receptor. J Biol Chem. 2002;277:46974–46979.
Stewart AK, Lassam NJ, Quirt IC, et al. Adenovector-mediated gene delivery of interleukin-2 in metastatic breast cancer and melanoma: results of a phase 1 clinical trial. Gene Ther. 1999;6:350–363.
Curiel DT . The development of conditionally replicative adenoviruses for cancer therapy. Clin Cancer Res. 2000;6:3395–3399.
Hitt MM, Graham FL . Adenovirus vectors for human gene therapy. Adv Virus Res. 2000;55:479–505.
Liu Y, Huang H, Saxena A, et al. Intratumoral coinjection of two adenoviral vectors expressing functional interleukin-18 and inducible protein-10, respectively, synergizes to facilitate regression of established tumors. Cancer Gene Ther. 2002;9:533–542.
St George JA . Gene therapy progress and prospects: adenoviral vectors [Review]. Gene Ther. 2003;10:1135–1141.
Liu Y, Zhang X, Zhang W, et al. Adenovirus-mediated CD40 ligand gene-engineered dendritic cells elicit enhanced CD8(+) cytotoxic T-cell activation and antitumor immunity. Cancer Gene Ther. 2002;9:202–208.
Ambar BB, Frei K, Malipiero U, et al. Treatment of experimental glioma by administration of adenoviral vectors expressing Fas ligand. Hum Gene Ther. 1999;10:1641–1648.
Parks R, Evelegh C, Graham F . Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther. 1999;6:1565–1573.
Trudel S, Li Z, Dodgson C, et al. Adenovector engineered interleukin-2 expressing autologous plasma cell vaccination after high-dose chemotherapy for multiple myeloma — a phase 1 study. Leukemia. 2001;15:846–854.
Wen XY, Mandelbaum S, Li ZH, et al. Tricistronic viral vectors co-expressing interleukin-12 (1L-12) and CD80 (B7-1) for the immunotherapy of cancer: preclinical studies in myeloma. Cancer Gene Ther. 2001;8:361–370.
Akbulut H, Zhang L, Tang Y, et al. Cytotoxic effect of replication-competent adenoviral vectors carrying L-plastin promoter regulated E1A and cytosine deaminase genes in cancers of the breast, ovary and colon. Cancer Gene Ther. 2003;10:388–395.
Ng P, Parks RJ, Cummings DT, et al. A high-efficiency Cre/loxP-based system for construction of adenoviral vectors. Hum Gene Ther. 1999;10:2667–2672.
Bangari DS, Mittal SK . Porcine adenoviral vectors evade preexisting humoral immunity to adenoviruses and efficiently infect both human and murine cells in culture. Virus Res. 2004, in press.
Zantek ND, Azimi M, Fedor-Chaiken M, et al. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 1999;10:629–638.
Price JE . Clonogenicity and experimental metastatic potential of spontaneous mouse mammary neoplasms. J Natl Cancer Inst. 1986;77:529–535.
Carles-Kinch K, Kilpatrick KE, Stewart JC, et al. Antibody targeting of the EphA2 tyrosine kinase inhibits malignant cell behavior. Cancer Res. 2002;62:2840–2847.
Addison CL, Braciak T, Ralston R, et al. Intratumoral injection of an adenovirus expressing interleukin 2 induces regression and immunity in a murine breast cancer model. Proc Natl Acad Sci USA. 1995;92:8522–8526.
Palmer K, Hitt M, Emtage PC, et al. Combined CXC chemokine and interleukin-12 gene transfer enhances antitumor immunity. Gene Ther. 2001;8:282–290.
Ding Y, Wen Y, Spohn B, et al. Proapoptotic and antitumor activities of adenovirus-mediated p202 gene transfer. Clin Cancer Res. 2002;8:3290–3297.
Vlachaki MT, Chhikara M, Aguilar L, et al. Enhanced therapeutic effect of multiple injections of HSV-TK + GCV gene therapy in combination with ionizing radiation in a mouse mammary tumor model. Int J Radiat Oncol Biol Phys. 2001;51:1008–1017.
Kikawa KD, Vidale DR, Van Etten RL, et al. Regulation of the EphA2 kinase by the low molecular weight tyrosine phosphatase induces transformation. J Biol Chem. 2002;277:39274–39279.
Graham FL, Smiley J, Russell WC, et al. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74.
Chen L, Anton M, Graham FL . Production and characterization of human 293 cell lines expressing the site-specific recombinase Cre. Somat Cell Mol Genet. 1996;22:477–488.
Graham FL,, Prevec L . Manipulation of adenovirus vectors. In: Murray EJ, ed. Methods of Molecular Biology: Gene Transfer and Expression Protocols. Totowa: Humana Press; 1991: 109–128.
Kinch MS, Kilpatrick KE, Zhong C . Identification of tyrosine phosphorylated adhesion proteins in human cancer cells. Hybridoma. 1998;17:227–235.
Graham FL, van der Eb AJ . A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467.
Sambrook J, Russell DW . Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York: Cold Harbor Press; 2001.
Acknowledgements
We thank Jane Stewart, Shaji Abraham, Rebecca Pratt, and Keith Kikawa for their technical advice, Elizabeth Bruckheimer for critical reading of the manuscript and Jane Kovach for excellent secretarial assistance. This work was partially supported by grants from Purdue Research Foundation, National Cancer Institute (U01 CA91318) and the US Army Medical Research Acquisition Activity.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noblitt, L., Bangari, D., Shukla, S. et al. Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1. Cancer Gene Ther 11, 757–766 (2004). https://doi.org/10.1038/sj.cgt.7700761
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700761
Keywords
This article is cited by
-
Novel approaches in cancer treatment: preclinical and clinical development of small non-coding RNA therapeutics
Journal of Experimental & Clinical Cancer Research (2021)
-
Oncogenic functions and therapeutic targeting of EphA2 in cancer
Oncogene (2021)
-
YSA-conjugated mesoporous silica nanoparticles effectively target EphA2-overexpressing breast cancer cells
Cancer Chemotherapy and Pharmacology (2018)
-
Cancer-associated fibroblasts promote gastric tumorigenesis through EphA2 activation in a ligand-independent manner
Journal of Cancer Research and Clinical Oncology (2018)
-
An integrated genomic approach identifies persistent tumor suppressive effects of transforming growth factor-β in human breast cancer
Breast Cancer Research (2014)