Abstract
Over the last years, evidence emerged demonstrating that the progression of renal fibrosis is reversible in experimental models. The present review summarizes the new insights concerning the mechanisms of progression and regression of renal disease and examines this novel evidence under the light of feasibility and transfer to human nephropathies. The involved mechanisms are discussed with particular emphasis on the fibrotic role of vasoactive peptides such as angiotensin II and endothelin, and growth factors such as transforming growth factor β (TGFβ). The possibility of regression is introduced by presenting the in vivo efficiency of anti-hypertensive treatments and of systems that antagonize the fibrogenic action of TGFβ such as bone morphogenic protein-7 (BMP-7) and hepatocyte growth factor. Finally, we provide a brief description of the promising future directions and clinical considerations about the applications of the experimental data to humans.
Similar content being viewed by others
Main
The number of patients with renal disease is growing worldwide. Actual estimation indicates that more than 500 millions of people suffer from renal disease. A significant part of these patients will progress to end-stage renal disease (ESRD). According to the American Society of Nephrology website, more than 500 000 patients need renal replacement therapy in US, which more than twice the number of ESRD cases for the year 1991.1 A similar trend is observed in Europe.2 According to Department of Health in France, 35 000 patients receive actually dialysis treatment and more than 25 000 have been transplanted. The frequency of cases is increasing with a rate of 7–8% per year. While dialysis is life sustaining and allows most patients to work and perform many normal activities, dependence on dialysis is strenuous, costly and is accompanied by severe dietary and lifestyle restrictions. In 2005, the direct medical payments for renal disease treatment was more than 2% of the total health expenses for France, making thus ESRD one of the most expensive diseases to treat on a per capita basis.
ESRD is characterized by a continuous decline of renal function owing to abnormal accumulation of extracellular matrix (mainly collagens type I and III) and to structural alterations in all renal compartments. Under normal conditions, there is a dynamic equilibrium between agents that promote the synthesis and stabilization of extracellular matrix and those that favour its degradation. In pathophysiological conditions that lead to the development of fibrosis, this equilibrium is altered due to exaggerated rates of extracellular matrix synthesis, to increasing capacity of extracellular matrix stabilization and/or to diminished rates of degradation. Since the progression of ESRD appears to display similar characteristics irrespective of the initiating cause and originating compartment, it is currently assumed that development of renal fibrosis follows a common pathway. For this reason, characterization and identification of profibrotic agents will provide important information for an efficient therapy against progression of ESRD.
The main puzzling question is to understand the conditions and the causes that make normal cells (such as smooth muscle, mesangial or tubular epithelial cells) to start producing abnormal extracellular matrix, to proliferate, to change phenotype and to become fibroblasts and to invade adjacent renal compartments.
To explain this puzzle a linear cascade-event hypothesis was initially proposed. In the case of hypertension-induced renal disease, for instance, it was advanced that increased systemic blood pressure induces local renal hemodynamic alterations under the reaction of vasoactive peptides like angiotensin II, which in turn induced inflammation and renal cell damage that was followed by exaggerated collagen accumulation. Under this view, fibrogenesis is a late event, an adaptive effort of the tissue secondary to the increased systemic blood pressure.
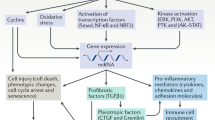
However, this conception was challenged over the last years by experimental models allowing to uncover that the so-called ‘classical’ vasoconstrictor agents such angiotensin II have a multitude of additional effects that can promote fibrosis independently of systemic hemodynamics. As an example, our laboratory demonstrated that angiotensin II could activate collagen I gene and induce renal vascular and glomerular fibrosis by at least two distinct pathways: one is acting through increase of renal endothelin synthesis followed by endothelin-mediated transactivation of the epidermal growth factor (EGF) receptor. The phosphorylation of EGF receptor leads to the activation of the MAPK 42/44 pathway and the formation of the AP-1 transcriptional complex.3, 4, 5, 6 Antagonists of endothelin or EGF receptors or inhibitors of MAPK phosphorylation cancelled the angiotensin II-induced activation of collagen I gene. Another way of angiotensin action on collagen I gene is mediated by the activation of the transforming growth factor β (TGFβ)/Smad pathway.7 This activation can be blocked by using anti-TGFβ antibodies, scavengers of TGFβ (such as decorin) or by activating the bone morphogenic proteins (BMPs) pathway (Figure 1). Both mechanisms are acting independently of systemic hemodynamics and have to occur simultaneously to induce collagen I gene activation.7 Other investigators provided evidence for additional actors such plasminogen activator inhibitor-1 (PAI-1), platelet-derived growth factor (PDGF), connective tissue growth factor, inflammatory cytokines as mediators of the angiotensin II-induced fibrosis.8, 9, 10 These studies clearly pointed out that the linear cascade-event hypothesis is not valid, at least for several experimental models, and has to be revisited and actualized. We propose that the different actions triggered by angiotensin II on cells (such contractility, inflammation, proliferation, apoptosis, extracellular matrix gene activation) are in close interaction with one another and according to the specific renal conditions (environmental, hormonal, homeostatic) can favor or not the development of fibrosis (Figure 2). Thus, the quest for the key mediator(s) involved and the discovery of eventual therapeutic target(s) can be more complicated than initially thought.11
Angiotensin II can induce in a simultaneous way a variety of effects on cells such as contractility, inflammation, proliferation, apoptosis or extracellular matrix gene activation, which are in close interaction with one to another and according to the specific renal conditions (environmental, hormonal, homeostatic) can favor or not favour the development of fibrosis
Reactive Oxygen Species
An emerging therapeutic strategy is to block the generation of free radicals, reactive oxygen species (ROS) and reactive nitrogen species (RNS). It is widely believed that oxidative stress and excessive generation of ROS, initiates and/or participates in a variety of vascular diseases. Because ROS induce endothelial damage and inflammation and alter renal vascular tone and capacity of electrolyte transport, it was suggested that they were involved in the progression of chronic renal disease.12 Indeed, in several experimental models, treatment with anti-oxidants gave impressive results of vascular and renal protection (regression of vascular remodeling, improvement of endothelial function, inhibition of inflammation, and decrease of blood pressure). However, the results of large-scale trials in patients with cardiovascular disease and/or diabetes were rather disappointing.13, 14 One option is that the current clinically available anti-oxidants lack the efficiency needed for long-term treatments. It is also possible that because ROS are mediators of vasoactive agents such as angiotensin II, targeting the initiator renin angiotensin system is sufficient to achieve decrease of ROS. In agreement with this notion, it has been recently reported that short-term treatment of hemodialyzed patients with classical anti-hypertensive agents (angiotensin receptor antagonist or calcium blocker) reduced the plasma levels of oxidative stress markers.15
Angiotensin II inhibition and renoprotection in human studies
After an initial observation that blockade of the angiotensin II action protected against the progression of renal failure in various renal diseases (such as glomerulopathies, interstitial nephritis, nephrosclerosis, polycystic kidney disease or diabetic nephropathy),16 large-scale studies concerning hypertensive patients with type II diabetes demonstrated a renoprotective efficiency of angiotensin II antagonism.17, 18, 19 In these studies, antagonism of AT1 angiotensin II receptor (ARB) reduced microalbuminuria and slowed the rate of decline of filtration rate; these renoprotective effects were independent of the blood-pressure decrease, supporting the notion of dissociation between systemic and local actions of angiotensin II. However, slower pace of the progression induced by angiotensin II antagonism although promising, is very far from an arrest of progression, or even better from a recovery of the renal disease.
Angiotensin II inhibition and renoprotection in animal studies
As mentioned in the introduction, the causes and the initiating mechanisms of chronic renal failure are multiple, varying from major metabolic or cardiovascular pathologies (diabetes, hypertension), to inflammatory or toxic aggressions or even to ‘physiological’ conditions such as aging. For these reasons several experimental models of renal disease have been developed trying to mimic the variety of initiating aggressions. As typical examples we mention the nitric oxide (NO) deficiency model, that is the equivalent of nephroangiosclerosis due to endothelial dysfunction, the model of unilateral ureteral obstruction (UUO) that mimics conditions of pyelonephritis, the anti-GMB model that reproduces conditions of glomerulonephritis and the 5/6 nephrectomy that reproduces conditions of severe reduction of functioning nephrons.
A spectacular recovery following treatment with ARB or angiotensin converting enzyme (ACE) inhibitors (Table 1) was reported in these experimental studies in contrast to clinical observations. In a model of hypertension-associated renal failure (NO deficiency model), renal function was severely reduced after 4–6 weeks with the association of increased levels of proteinuria and of plasma creatinine, an index of decreased glomerular filtration rate, and a mortality rate accounted for 20%.20 The impairment of renal function was accompanied by an exaggerated expression of TGF-β, collagen I and collagen IV within the renal vasculature and an abnormal accumulation of extracellular matrix in glomeruli. In addition, activities of matrix metalloproteinases (MMP) -2 and -9 were increased several-fold in glomeruli. At this phase of disease, administration of an angiotensin II receptor antagonist for 1 month normalized cellular phenotype, histology and functional parameters of kidney indicating that the progression of renal vascular fibrosis was a reversible process. The proposed mechanism of the regression was dual: inhibition of the exaggerated synthesis of extracellular matrix (owing to blockade of angiotensin II-TGFβ pathway) and increased rate of matrix degradation (owing to metalloproteinases activity).20 Subsequent studies confirmed the reversibility of fibrotic process after angiotensin II blockade in the model of acute nephronic reduction.21, 22 Eight weeks after surgery, animals were treated for 1 month with an ACE inhibitor. Morphological evaluation indicated regression of pre-existing glomerular, tubular, and vascular lesions, and reversal of glomerular hypertrophy. The decreased number of podocytes, following renal ablation, was not restored by the pharmacological treatment suggesting that glomerular regeneration largely depended on the degree of damage of glomerular podocytes.
What explanation can be given for this pronounced difference of the efficiency of angiotensin II antagonism against renal disease between humans and rodents? One option is that in experimental models the kinetics concerning the development of fibrosis is faster (weeks, few months at best) compared to humans (years). Therefore, it is possible that the treatment in animals have started relatively early, before the irreversible destruction of the renal structure (before reaching a no-return point). Another explanation (not excluding the first) is that the disease is more complex in humans than in rodents and treatments that have spectacular curative effects in rodents do not work with the same efficiency in humans because of a much more multifactorial and complex pathology. A paradigm for this kind of situation is tumor development and therapy: several drugs working in rodents have a limited efficacy in humans. Thus, it is possible that in humans several treatments have to be combined or added to classical anti-hypertensive therapy to obtain regression of fibrosis and reversal of renal disease. These potential targets can be either mediators of the angiotensin action, systems degrading extracellular matrix proteins or participating in the stabilization of extracellular matrix.
Mediators of the Angiotensin II Action
Plasminogen activator inhibitor-1
Increased levels of PAI-1, an enzyme inhibiting the plasminogen proteolysis to plasmin, have been observed in experimental nephropathies or during aging.23, 24 Moreover, deletion of PAI-1 gene protected animals from the development of vascular or renal interstitial fibrosis.25, 26 The activation of PAI-1 during fibrotic process appears to be due to the angiotensin II action and treatment with angiotensin inhibitors or receptor antagonists was accompanied by decreased levels of PAI-1 within the kidney.27 On the basis of these results, several investigators have proposed to inhibit PAI-1 as a mean to treat renal fibrosis. However, PAI-1 null mice displayed a worse degree of renal failure in the anti-GBM (glomerular basal membrane) model, indicating that PAI-1 plays a more complex role.28 So it is possible that PAI-1 can mediate opposite effects depending on the model and/or the phase of the evolution of the disease.
Endothelin
Endothelin-1 is also an important mediator of vascular and renal fibrosis3, 29, 30 (Figure 1). Studies performed in a variety of experimental models demonstrated that endothelin gene and/or peptide expression were increased during nephropathy and colocalized with fibrotic lesions. In addition, pharmacological antagonism of endothelin receptors delayed the evolution and/or prevented renal failure. We investigated the efficiency of endothelin receptor antagonism to treat renal failure in the NO-deficiency model. Endothelin receptor antagonism, introduced after the appearance of fibrotic lesions, reduced mortality (Table 1); moreover, the treated animals displayed a less severe degree of glomerular lesions even compared with those at the beginning of the treatment, suggesting thus that renal vascular fibrosis could regress by ET-1 antagonism.31 However, this regression was partial and less efficient compared to the consequences of angiotensin II antagonism.
Transactivation of tyrosine kinase growth factor receptors
Nuclease-resistant high-affinity aptamers that neutralized the effects of PDGF-B inhibited glomerular and interstitial fibrosis in a rat model of mesangioproliferative glomerulosclerosis.32 We tested the interaction between vasoconstrictors, EGF receptor transactivation and collagen I gene, and we found that endothelin induced a rapid phosphorylation of EGF receptor that mediated collagen I gene activation in renal cortex. Moreover, we observed that EGF receptor was activated within glomeruli concomitantly to the development of glomerulosclerosis in the NO-deficiency model. Use of an EGF receptor-tyrosine kinase inhibitor in a preventive way, normalized the MAPK activation, inhibited the abnormal increase of collagen I gene expression, decreased proteinuria and creatininemia and prevented the development of renal vascular and glomerular fibrosis.6, 33 From these studies, it appears that blockade or antagonism of growth factor receptors offer an interesting choice as therapy against fibrosis. However, the experience from human trials with long-term blockade of growth factor action indicates several undesired secondary effects. Since renal fibrosis requires a life-long treatment, it appears more realistic to combine short periods of growth factor inhibition to a ‘traditional’ chronic renoprotective therapy. An alternative would be to identify the ligands of the growth factor receptors activated during the course of nephropathy (e.g., TGFα or HB-EGF for the EGF receptor) and to block specifically their availability.
Antagonists of TGFβ action: BMP-7
TGFβ is an agent promoting extracellular matrix synthesis and is considered to play a major role as mediator of the fibrogenic action of several vasoconstrictor peptides, especially that of angiotensin II. The initially proposed strategies to block the action of TGFβ at the systemic (decorin, blocking antibody) or receptor (soluble receptors) level presented important limitations (increased concentrations of decorin lack the anti-TGFβ specificity and can produce secondary effects, while the cost and the way of delivery – intravenous infusion – of soluble receptors make unrealistic a long-term treatment) not permitting the transfer to human therapy.
As alternative strategy was proposed to block the fibrogenic action of TGFβ with agents targeting its signaling pathway such as the BMP-7. Systemic administration of recombinant human BMP-7 led to repair severely damaged renal tubular epithelial cells and to improve renal function and survival in mice with nephrotoxic serum nephritis (Table 1) and in two genetic models for chronic renal injury and fibrosis (mice deficient in the α3-chain of type IV collagen and MRL/MpJlpr/lpr lupus mice).34, 35 Although these results indicate the potential of BMP-7 to reverse the TGFβ-induced injury and to repair renal tissue in a variety of experimental models, the way of the administration of BMP-7 (intraperitoneal injections daily) and the potential side effects of a long-term administration of a bone morphogenic factor limit, at least for the moment, the transfer of this approach to humans.
Antagonists of TGFβ action: HGF
Hepatocyte growth factor (HGF) is another endogenous peptide antagonizing the effects of TGFβ. The potential of HGF as antifibrotic agent has been recently reviewed by Liu and Yang.36 In the UUO and the rat remnant kidney, models of renal interstitial fibrosis, administration of recombinant HGF retarded the progression of renal lesions by blunting the myofibroblast accumulation and collagen deposition within the kidney.37, 38 This action of HGF was attributed to a MAPK-dependent blockade of TGFβ activity; in contrast, blocking of endogenous HGF by an anti-HGF-neutralizing antibody increased interstitial collagen and aggravated the degree of renal fibrosis. More recently, treatment with human HGF, in the onset of fibrogenic mechanisms, reduced renal failure and mortality, diminished tubulo-interstitial damage, induced cell regeneration, decreased inflammation and prevented late interstitial fibrosis and glomerulosclerosis in a rat model of chronic allograft nephropathy.39 Although promising, the above mentioned approaches were preventive with treatments started before or at the beginning of the disease; no data are available showing a therapeutic effect with HGF administered after the establishment of chronic renal failure.
Kallikrein
The effect of kallikrein and activation of the kinin B2 receptor on the reversal of salt-induced inflammation and renal fibrosis in Dahl salt-sensitive (DSS) rats has been recently investigated (Bledsoe et al.40; Table 1). Four weeks after high-salt loading, when renal injury was apparent, adenovirus harboring the human tissue kallikrein gene was injected into DSS rats. Two weeks after adenovirus injection, salt-induced glomerular sclerosis, tubular protein cast formation, and monocyte/ macrophage accumulation in the kidney were notably reversed by kallikrein. Decreased intercellular adhesion molecule-1 expression paralleled this observation. Kallikrein gene delivery also dramatically reduced collagens I, III and IV and reticulin deposition, accompanied by a decline in myofibroblast accumulation and TGFβ expression. Moreover, kallikrein reversed salt-induced glomerular hypertrophy and diminished urinary protein and blood urea nitrogen levels. Furthermore, kallikrein gene delivery restored nitric oxide production and suppressed NADH oxidase activity and superoxide generation.
Agents degrading Extracellular Matrix: Metalloproteinases
As discussed earlier, data from our laboratory indicated that the activation of MMPs played a beneficial role against the development of renal fibrosis in the NO deficiency model of renal disease.20 These results are corroborated by the observation that most often in experimental models and human diseases in which ECM accumulation is observed, fibrosis is accompanied by sustained increase of MMP expression. In our view, the upregulated MMP expression reflects cellular compensatory mechanisms aimed at limiting the rate of matrix accumulation. However, other investigators showed that active MMP-2 is required for the mesenchymal transformation of renal epithelial cells in vitro.41 In addition, mice specifically overexpressing constitutively active MMP-2 in the renal proximal tubule displayed the whole spectrum of pathological and functional changes characteristic of human chronic kidney disease. Early activation of MMP-2 was accompanied by structural alterations in the tubular basement membrane, a process that triggers tubular epithelial-to-mesenchymal transformation, with resultant tubular atrophy, fibrosis and renal failure.42
This apparent controversy in the understanding the role of MMPs in the progression of renal disease could be explained by the recent observations made in an animal model (mice deficient in the α3 chain of type-IV collagen) mimicking a genetic form of chronic renal failure (Alport's syndrome).43 In this model, MMP-2, MMP-3 and MMP-9 levels were significantly upregulated in glomeruli early, before the onset of proteinuria, whereas MMP expression spread from the glomeruli into the tubulointerstitial compartment only after the establishment of renal disease in the glomerulus and the progression towards tubulointerstitial fibrosis. Pharmacological inhibition of enzymatic activity of MMPs before the onset of proteinuria and GBM structural defects, led to significant improvement in disease progression associated with delayed proteinuria and markedly prolonged survival. In contrast, inhibition of MMPs after induction of proteinuria led to acceleration of disease associated with extensive interstitial fibrosis and early death.
It appears, thus, that activation of MMPs can be either beneficial or detrimental to renal function depending on the compartment and the phase of renal disease. This observation makes problematic the use of agents targeting matrix-degrading enzymes as therapeutic tools, at least up to the time that we will get a better understanding and control of their activity in the different segments of the kidney.
Collagen Receptors: Discoidin Domain Receptor 1
The above mentioned studies focused mainly in the systems or agents that promote or degrade extracellular matrix synthesis. Less is known about the mechanisms regarding the post-synthesis regulation of extracellular matrix, such as matrix anchoring and interactions with the cell membrane. Among the systems that interact with the extracellular matrix is the discoidin domain receptor 1 (DDR1). The interesting feature of DDR1 is that after the binding of collagens, this receptor is dimerized, tyrosine-kinase phosphorylated and this phosphorylation leads to P38MAP/kinase activation, making it the only known collagen receptor displaying intracellular signalling activity.44 In addition, in vitro studies indicated that DDR1 could be a major mediator of the inflammatory response because it is essential for the maturation and differentiation of monocytes to macrophages.45, 46 To test the role of DDR1 in the progression of renal failure, we examined the development and the severity of renal vascular and glomerular lesions in DDR1 null mice in an experimental model of hypertension-induced renal disease (angiotensin II infusion).47 After 4 or 6 weeks of angiotensin II administration, wild-type mice developed hypertension associated to perivascular inflammation, glomerular sclerosis and proteinuria. Systolic pressure increase was similar in the DDR1-deficient mice, but the histological lesions of glomerular fibrosis and inflammation were significantly blunted and proteinuria was markedly prevented. Immunostaining for lymphocytes, macrophages and abnormal accumulation of collagens I and IV were prominent in the renal cortex of wild-type animals, but negligible in DDR1 null mice. In addition, renal cortical slices of DDR1 null mice showed a blunted response of chemokines to LPS that was accompanied by a considerable protection against the LPS-induced mortality.
We propose that DDR1 participates in fibrosis as an amplifier of the angiotensin II-induced collagen synthesis. The extracellular part of the DDR1 is responsible for binding the collagen. Once collagen is bound, the intracellular part of the receptor is activated and leads to stimulation of the P38MAP/kinase pathway and induction of the inflammatory response (Figure 3). After the inflammation is triggered, it activates collagen synthesis, facilitates collagen binding, which in turn increases inflammation and so on. As a result, this positive feedback leads to the development of fibrosis.
Mechanism of DDR1 action on renal vasculature: collagen binds to the extracellular part of DDR1, phosphorylates the intracellular part of the receptor and leads to stimulation of the P38MAP/kinase pathway and induction of the inflammatory response. Once inflammation is triggered, it promotes in turn collagen synthesis, which is bound on DDR1 and further increases inflammation and so on. As a result, this positive feedback leads to the development of fibrosis
Conclusion: Future Directions
We believe that cellular events like vascular contractility, inflammation or fibrogenesis are adaptive responses occurring locally to permit to the kidney to adjust and continue its function in presence of the different aggressions (Figure 2). When these events occur simultaneously (such as during hypertension-induced renal inflammation and fibrosis), they cooperate, amplify the effect of each other and lead to the progression of renal disease. The experience from the clinical trials shows that since angiotensin II blockers can slow down but not reverse the decline of renal function, they have to be associated with an additional therapy. The recent observations using inhibitors of the different mediators of angiotensin II effects (like endothelin-1, PAI-1, TGFβ, growth factor receptors) show several undesirable effects causing, at least at the present time, their use to humans problematic. Similarly, antifibrotic agents (such as exogenous BMP-7) or inhibitors of systems degrading extracellular matrix proteins (like MMPs) lack for the moment, the spatial and temporal specificity and can create adverse secondary complications. We believe that a beneficial option would be to block to systems like DDR1, whichthat are common to proliferative, fibrogenic and inflammatory pathways. Identifying and targeting these mediators will allow to develop therapeutic agents that would provide a valuable assistance to ‘classical’ therapy antagonizing RAS to achieve regression of renal fibrosis and reversal of renal failure. These therapies should be started as early as possible, before a no-return point of the decline of renal function is reached. The search of easily detectable markers (in urine or plasma) detecting and predicting the evolution of renal function towards ESRD will be precious for the patients, actual or – unfortunately – to come.
Abbreviations
- BMP-7:
-
bone morphogenic protein
- DSS:
-
dahl salt-sensitive
- EGF:
-
epidermal growth factor
- ESRD:
-
end-stage renal disease
- HGF:
-
hepatocyte growth factor
- PAI-1:
-
plasminogen activator inhibitor-1
- PDGF:
-
platelet-derived growth factor
- TGFβ:
-
transforming growth factor β
References
Incidence and prevalence of ESRD##United States Renal Data System. Am J Kidney Dis 1998; 32 (Suppl 1): S38–S49.
Valderrabano F, Berthoux FC, Jones EH et al. Report on management of renal failure in Europe, XXV, 1994 end stage renal disease and dialysis report. The EDTA-ERA Registry. Nephrol Dial Transplant 1996; 11 (Suppl 1): 2–21.
Chatziantoniou C, Boffa JJ, Ardaillou R, Dussaule JC . Nitric oxide inhibition induces early activation of type I collagen gene in renal resistance vessels and glomeruli in transgenic mice: role of endothelin. J Clin Invest 1998; 101: 2780–2789.
Boffa JJ, Tharaux PL, Placier S, Ardaillou R, Dussaule JC, Chatziantoniou C . II activates collagen type I gene in the renal vasculature of transgenic mice during inhibition of nitric oxide synthesis: evidence for an endothelin-mediated mechanism. Circulation 1999; 100: 1901–1908.
Tharaux PL, Chatziantoniou C, Fakhouri F, Dussaule JC . Angiotensin II activates collagen I gene through a mechanism involving the MAP/ER kinase pathway. Hypertension 2000; 36: 330–336.
Flamant M, Tharaux PL, Placier S, Henrion D, Coffman T, Chatziantoniou C et al. Epidermal growth factor transactivation mediates the tonic and fibrogenic effects of endothelin in the aortic wall of transgenic mice. FASEB J 2003; 17: 327–329.
Fakhouri F, Placier S, Ardaillou R, Dussaule JC, Chatziantoniou C . Angiotensin II activates collagen type I gene in the renal cortex and aorta of transgenic mice through interaction with endothelin and TGF-beta. J Am Soc Nephrol 2001; 12: 2701–2710.
Berk BC . Angiotensin II signal transduction in vascular smooth muscle: pathways activated by specific tyrosine kinases. J Am Soc Nephrol 1999; 10 (Suppl 11): S62–S68.
Fogo AB . Renal fibrosis and the renin-angiotensin system. AAdv Nephrol Necker Hosp 2001; 31: 69–87.
Klahr S, Morrissey JJ . The role of vasoactive compounds, growth factors and cytokines in the progression of renal disease. Kidney Int Suppl 2000; 75: S7–S14.
Ruiz-Ortega M, Esteban V, Ruperez M, Sanchez-Lopez E, Rodriguez-Vita J, Carvajal G et al. Renal and vascular hypertension-induced inflammation: role of angiotensin II. Curr Opin Nephrol Hypertens 2006; 15: 159–166.
Modlinger PS Wilcox CS Aslam S . Nitric oxide, oxidative stress, and progression of chronic renal failure. Semin Nephrol 2004; 24: 354–365.
Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999; 354: 447–455.
Yusuf S, Sleight P, Poque J, Bosch J, Dussaule JC, Dagenais G et al. Vitamin E supplementation and cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000; 342: 154–160.
Aslam, Santha T, Leone A, Wilcox C . Effects of amlodipine and valsartan on oxidative stress and plasma methylarginines in end-stage renal disease patients on hemodialysis. Kidney Int 2006; 70: 2109–2115.
Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The angiotensin-converting-enzyme inhibition in progressive renal insufficiency study group. N Engl J Med 1996; 334: 939–945.
Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860.
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869.
Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P . The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345: 870–878.
Boffa JJ, Ying L, Placier S, Stefanski A, Dussaule JC, Chatziantoniou C . Regression of renal vascular and glomerular fibrosis: role of angiotensin II receptor antagonism and metalloproteinases. J Am Soc Nephrol 2003; 14: 1132–1144.
Adamczak M, Gross ML, Amann K, Ritz E . Reversal of glomerular lesions involves coordinated restructuring of glomerular microvasculature. J Am Soc Nephrol 2004; 15: 3063–3072.
Adamczak M, Gross ML, Krtil J, Koch A, Tyralla K, Amann K et al. Reversal of glomerulosclerosis after high-dose enalapril treatment in subtotally nephrectomized rats. J Am Soc Nephrol 2003; 14: 2833–2842.
Eddy A, Giachelli CM . Renal expression of genes that promote interstitial inflammation and fibrosis in rats with protein-overload proteinuria. Kidney Int 1995; 47: 1546–1557.
Ma LJ, Nakamura S, Whitsitt JS, Marcantoni C, Davidson JM, Fogo AB . Regression of sclerosis in aging by an angiotensin inhibition-induced decrease in PAI-1. Kidney Int 2000; 58: 2425–2436.
Kaikita K, Fogo AB, Ma L, Schoenhard JA, Brown NJ, Vaughan DE . Plasminogen activator inhibitor-1 deficiency prevents hypertension and vascular fibrosis in response to long-term nitric oxide synthase inhibition. Circulation 2001; 104: 839–844.
Oda T, Jung YO, Kim HS, Cai X, Lopez-Guisa JM, Ikeda Y et al. PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int 2001; 60: 587–596.
Eddy AA, Fogo AB . Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol 2006; 17: 2999–3012.
Hertig A, Berrou J, Allory Y, Breton L, Commo F, Costa De Beaureqard MA et al. Type 1 plasminogen activator inhibitor deficiency aggravates the course of experimental glomerulonephritis through overactivation of transforming growth factor beta. FASEB J 2003; 17: 1904–1906.
Benigni A, Zoja C, Corna D, Orisio S, Facchinetti D, Benati L et al. Blocking both type A and B endothelin receptors in the kidney attenuates renal injury and prolongs survival in rats with remnant kidney. Am J Kidney Dis 1996; 27: 416–423.
Hocher B, Thöne-Reineke C, Rohmeiss P, Schmager F, Slowinski T, Burst V et al. Endothelin-1 transgenic mice develops glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J Clin Invest 1997; 99: 1380–1389.
Boffa JJ Tharaux PL, Dussaule JC, Chatziantoniou C . Regression of renal vascular fibrosis by endothelin receptor antagonism. Hypertension 2001; 37: 490–496.
Ostendorf T Kunter U, Grone HJ, Bahlmann F, Kawachi H, Shimizu F et al. Specific antagonism of PDGF prevents renal scarring in experimental glomerulonephritis. J Am Soc Nephrol 2001; 12: 909–918.
François H, Placier S, Flamant M, Tharaux PL, Chansel D, Dussaule JC et al. Prevention of renal vascular and glomerular fibrosis by epidermal growth factor receptor inhibition. FASEB J 2004; 18: 926–929.
Zeisberg M, Bottiglio C, Kumar N, Maeshima Y, Strutz F, Muller GA et al. Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am J Physiol Renal Physiol 2003; 285: F1060–F1067.
Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R . BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 2003; 9: 964–968.
Liu Y, Yang J . Hepatocyte growth factor: new arsenal in the fights against renal fibrosis? Kidney Int 2006; 70: 238–240.
Yang J, Dai C, Liu Y . Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction. Am J Pathol 2003; 163: 621–632.
Yang J, Liu Y . Delayed administration of hepatocyte growth factor reduces renal fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 2003; 284: F349–F357.
Herrero-Fresnada, Torras J, Franquesa M, Vidal A, Cruzado JM, Lloberas N et al. HGF gene therapy attenuates renal allograft scarring by preventing the profibrotic inflammatory-induced mechanisms. Kidney Int 2006; 70: 265–274.
Bledsoe G, Shen B, Yao Y, Zhang JJ, Chao L, Chao J . Reversal of renal fibrosis, inflammation, and glomerular hypertrophy by kallikrein gene delivery. Hum Gene Ther 2006; 17: 545–555.
Cheng S, Lovett DH, Gelatinase A . (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am J Pathol 2003; 162: 1937–1949.
Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH . Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. FASEB J 2006; 20: 1898–1900, (E-pub 2006 August 4).
Zeisberg M, Khurana M, Rao VH, Cosgrove D, Rougier JP, Werner MC et al. Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med 2006; 3: e100.
Vogel W, Gish GD, Alves F, Pawson T . The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell 1997; 1: 13–23.
Matsuyama W, Faure M, Yoshimura T . Activation of discoidin domain receptor 1 facilitates the maturation of human monocyte-derived dendritic cells through the TNF receptor associated factor 6/TGF-beta-activated protein kinase 1 binding protein 1 beta/p38 alpha mitogen-activated protein kinase signaling cascade. J Immunol 2003; 171: 3520–3532.
Matsuyama W, Kamohara H, Galligan C, Faure M, Yoshimura T . Interaction of discoidin domain receptor 1 isoform b (DDR1b) with collagen activates p38 mitogen-activated protein kinase and promotes differentiation of macrophages. FASEB J 2003; 17: 1286–1288.
Flamant J, Placier S, Rodenas A, Curat CA, Vogel WF, Chatziantoniou C et al. Discoidin domain receptor 1 null mice are protected against hypertension-induced renal disease. J Am Soc Nephrol 2006; 17: 3374–3381.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by P Nicotera
Rights and permissions
About this article
Cite this article
Dussaule, JC., Chatziantoniou, C. Reversal of renal disease: is it enough to inhibit the action of angiotensin II?. Cell Death Differ 14, 1343–1349 (2007). https://doi.org/10.1038/sj.cdd.4402143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4402143
Keywords
This article is cited by
-
Targeting connexin 43 protects against the progression of experimental chronic kidney disease in mice
Kidney International (2014)
-
Bone marrow mononuclear cells attenuate fibrosis development after severe acute kidney injury
Laboratory Investigation (2010)
-
Reversal of proteinuric renal disease and the emerging role of endothelin
Nature Clinical Practice Nephrology (2008)
-
Janus a god with two faces: death and survival utilise same mechanisms conserved by evolution
Cell Death & Differentiation (2007)
-
Pathobiology of transforming growth factor β in cancer, fibrosis and immunologic disease, and therapeutic considerations
Laboratory Investigation (2007)