Abstract
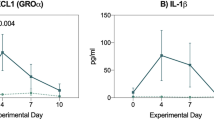
Intensive cytotoxic therapy with bone-marrow transplantation (BMT) allows a potential cure for haematological malignancies. Protective strategies to minimise haematological toxicities have been successful and currently toxicity to the gastro-intestinal tract is the major cause of treatment-related morbidity and the dose-limiting factor that prevents further dose escalation. In a randomised, placebo-controlled trial we investigated whether an oral immunoglobulin preparation (IgA-IgG) can diminish intestinal toxicity with autologous BMT. IgA-IgG (n = 6) and placebo (n = 7) were orally administered from 1 day prior to the start until 1 week after the termination of the cytotoxic treatment (a total of 14 days). Intestinal toxicity was assessed by a51<Cr-EDTA absorption test for intestinal permeability and by the clinical criteria laid down by the WHO for the period before the start of the cytotoxic treatment, 1 day prior to stem-cell infusion and 4, 7, 10 and 14 days after stem-cell infusion. In the placebo group there was a significant increase in intestinal permeability on day 4 (P < 0.005) and on day 7 (P < 0.05) after stem-cell infusion, compared with the baseline, which was not seen for iga-igg. in addition, patients receiving iga-igg had significantly less intestinal permeability on day 4 (P < 0.05) and on day 7 (P < 0.05), compared with the placebo group. no significant, positive effect as regards clinical toxicity was observed. oral administration of iga-igg to patients undergoing intensive cytotoxic therapy prior to bmt seems to have a protective effect on the gut mucosa barrier which is normally disrupted by this therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Johansson, JE., Ekman, T. Gut mucosa barrier preservation by orally administered IgA-IgG to patients undergoing bone marrow transplantation: a randomised pilot study. Bone Marrow Transplant 24, 35–39 (1999). https://doi.org/10.1038/sj.bmt.1701821
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1701821