Abstract
The efficacy of olfactory mucosal autografts (OMAs) for chronic spinal cord injury (SCI) has been reported, but there is no report documenting electrophysiological conductivity via the emergence of motor evoked potentials (MEPs). We report the case of a 39-year-old man with chronic, complete SCI at T8, who exhibited MEPs after OMA transplantation, and, with intensive rehabilitation, was ultimately able to ambulate with short leg braces and Lofstrand crutches. The initial injury occurred in a motor vehicle accident in November 1999 and resulted in a complete loss of sensorimotor function below T8. OMA transplantation to the injury site was performed in March 2010 in combination with intensive pre- and postoperative rehabilitation. The patient exhibited voluntary electromyograph (EMG) activity and MEPs at 96 and 144 weeks after transplantation and he was was ambulatory with short leg braces and Lofstrand crutches at 144 weeks after transplantation. We were able to elicit MEPs after OMA with intensive rehabilitation. To our knowledge, this is the first report of recovery of electrophysiological conductivity in the spinal cord after any type of treatment for chronic, complete SCI.
Similar content being viewed by others
The olfactory mucosa is an excellent source of autologous adult neuronal precursor cells. The neurons and sustentacular cells of the olfactory mucosa continuously renew themselves throughout life by proliferation of basal global stem cells.1,2 The ensheathing cells of the olfactory mucosa have gained much attention because of their potential application in the repair of SCI.3,4 Olfactory tissue is easily accessible and can be obtained by a simple biopsy performed through the external nares.5
Recent studies of spinal cord axonal regeneration have produced good long-term results using various types of tissue scaffolds,6,7 and we have previously reported that olfactory mucosa grafts were effective in restoring functional recovery in rats following spinal cord transection, with histological evidence of neuronal regeneration.8–10 In a clinical trial of OMA in humans with chronic traumatic SCI, Lima et al. reported restoration of voluntary EMG responses in 15 of 20 patients (75%) with mean American Spinal Injury Association motor score improvements of 4.95±7.1 points during a mean follow-up period of 27.7 months.11 However, these authors did not examine recovery of electrophysiological conductivity by MEP.
Here, we report a case of MEP detection after OMA accompanied by intensive rehabilitation.
A 39-year-old man sustained a T8 spinal cord injury (SCI) in a motor vehicle accident in November 1999. He presented with a complete loss of sensorimotor function below T8. At the time of the injury, he received emergency treatment and standard rehabilitation followed by additional locomotor training. The patient was referred to our hospital in April 2009. After nearly 10 years of gait and standing training with long leg braces, he had no contraction of the leg muscles or anal sphincter and no sensation below T8. His neurological deficit was American Spinal Injury Association (ASIA) Impairment Scale (AIS) A. There was no EMG response in the leg muscles during leg-upward tasks, and transcranial motor evoked potential (MEP) elicited no leg muscle response. Magnetic resonance imaging showed an injured cord segment of 2.94 cm long with myelomalacia and atrophy (Figures 1a and b). The patient was scheduled for olfactory mucosa autograft (OMA) transplantation to the injury site. He underwent intensive in-hospital rehabilitation for 2 months before transplantation, during which his neurological deficit did not improve. The rehabilitation schedule is shown in Table 1.
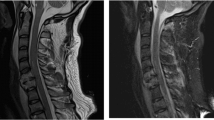
Magnetic resonance imaging (MRI). (a) T1-weighted sagittal image before transplantation shows atrophic change of the thoracic spinal cord. (b) T2-weighted sagittal image before transplantation shows an intramedullary high-intensity area. MRI at 48 weeks after transplantation shows fairly complete filling of cavities with heterogeneous intensity on T1- (c) and T2-weighted (d) images. (e) Gadolinium-enhanced images also show heterogeneous enhancement of the grafts. No evidence of neoplastic tissue overgrowth was observed during the initial follow-up period.
The surgery was performed in March 2010. Glial scar tissue was surgically resected from the injured cord after laminectomy and the OMA was transplanted to the injury site. The olfactory mucosa was removed under endoscopy and grated into the lesion site at the time of surgery, without prior cell or tissue culture. To avoid contamination with respiratory mucosa, olfactory mucosa was taken from the area of the upper nasal cavity where the olfactory nerve sensory fibers pass through perforations in the cribriform plate. Microbiological specimens were taken from the olfactory mucosa before and during the operation. The patient resumed rehabilitation (6.5 h per day and about 40 h per week) at 2 weeks after transplantation. Rehabilitation consisted of passive and assisted range of motion and strengthening exercises, functional training for balance, posture, and standing, and gait activities (Table 1). At 48 weeks, EMG biofeedback training and other measures described below were added to the rehabilitation program.
The safety and efficacy measures for OMA transplantation at our institution are listed in Table 2. Neurological examinations were performed preoperatively and at 4, 12, 24, 36, 48, 96 and 144 weeks after OMA and the patient was evaluated for MEPs at 96 and 144 weeks, respectively. Preoperative and postoperative examinations included ASIA neurological assessment in accordance with the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI),12 standard EMG with recordings during voluntary muscle movements, somatosensory evoked potentials recorded cortically after tibial nerve stimulation, urodynamic studies, full spinal cord magnetic resonance imaging and otolaryngological evaluation including a general ear, nose and throat examination, nasal endoscopy, and olfactory evaluation, and computed tomography of the nose and paranasal sinuses. No serious adverse events were reported in the present case.
MEP response to bifocal transcranial magnetic stimulation was evaluated bilaterally in the rectus femoris muscles. TMS was performed using a 7-cm diameter coil from MagVenture A/S, Denmark (MagPro 100), and navigation-guided TMS (Brainsight Frameless 1.5, Rogue Research Inc., Montreal, Canada) was used to determine the optimal position of each stimulation point (stimulation hot spots), starting about 4 cm rostral to Cz (vertex).13 TMS was delivered every 5–6 s. The duration of the monophasic transcranial single-pulse stimulus was 100 μs, the sample frequency was 2000 Hz, and a band-pass filter was set at 30 Hz–1 kHz. When the patient was unable to produce force, he was asked to exert as much volitional stimulation as possible. If there was a well-defined response, 3–5 representative MEPs at the desired stimulus intensity were recorded, and when there was a visible but poorly defined muscle response, up to 10 stimuli were delivered in order to realize three responses that could be stored offline for further analysis.14,15 The onset of the fastest response from four repeated MEP trials was identified as the onset latency, MEP amplitude was calculated from baseline to the negative peak for the largest response out of four trials, and the intensity of the magnetic stimulus was expressed as a percentage of the maximal stimulator output.
The patient did not have any notable improvement in his ASIA sensory score, and we could not elicit somatosensory evoked potentials responses from the tibial nerve. There was no urological improvement during the follow-up period. However, at 24 weeks the patient’s ASIA motor score had improved from 50 to 52, and it further improved to 56 at 144 weeks (Figure 2).
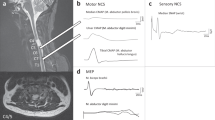
At 12 weeks, the quadriceps, hamstrings, anterior tibialis and gastrocnemius muscles produced EMG responses during the leg-upward task. By 36 weeks, the patient was able to crawl on his hands and knees and at 48 weeks he was ambulating with long leg braces and Lofstrand crutches. We did not detect EMG signals in the leg during walking, but we did detect EMG signals bilaterally upon voluntary contraction of the quadriceps, hamstrings, anterior tibialis, gastrocnemius, gluteus and thenar muscles (Figure 3).
The patient received EMG biofeedback training and practiced knee walking in a warm-water pool from weeks 48 to 96 after transplantation. The goal of the EMG biofeedback training was to identify triggers of voluntary action by detecting neuromuscular contractions and providing feedback signals to the patient in order to establish learned voluntary control.16 During this training, surface electrodes were placed over the quadriceps muscles and the patient was instructed to attempt knee extension while watching the EMG signals on a monitor. By 96 weeks, he was able to extend his knees on his own and to walk on his knees in the parallel bars, and MEPs were elicited in both quadriceps muscles at 96 and 144 weeks, respectively (Figure 4). The patient used iliopsoas for thigh flexion in the swing phase, but he did not use the hamstrings for knee flexion. He could not push off his toes because he had no use of tibialis anterior or gastrocnemius. At 144 weeks, the patient was ambulatory with short leg braces and Lofstrand crutches. The patient’s condition was documented pre- and postoperatively, and this is shown in a video recording that accompanies this report.
In terms of the SCI, magnetic resonance imaging at 48 weeks after OMA showed fairly complete filling of cavities with heterogeneous signal intensities on T1- and T2-weighted images. Gadolinium-enhanced magnetic resonance imaging also showed heterogeneous enhancement of the graft. No evidence of neoplastic tissue overgrowth was observed (Figures 1c–e).
Elicitation of MEPs and improved ambulation have not been observed in any previously reported treatment for patients with sensorimotor-complete SCI.17 In the present case, as suggested by elicitation of MEPs in the quadriceps muscles, OMA, in combination with intensive rehabilitation and EMG biofeedback training, facilitated restoration of severely damaged neural circuits in a patient with chronic sensorimotor-complete SCI.
This patient was a participant in an ongoing clinical trial of OMA transplantation for patients with chronic SCI at our institution (eight patients in total). We are reporting this case after an adequate follow-up period because we believe that OMA transplantation combined with intensive rehabilitation, including EMG biofeedback training, has the potential to provide further advances in treatment for patients with chronic, complete SCI.
Information about OMA derived from the studies of Lima et al. has been invaluable to basic and clinical researchers who are investigating regeneration in chronic SCI. Their pioneering clinical trials have demonstrated that OMA transplantation is ‘feasible, relatively safe, and potentially beneficial.’11,18 OMA is advantageous in that it involves transplantation of whole tissue that is rich in factors associated with neuronal regeneration. To achieve significant functional reconstruction of the spinal cord after SCI, it is necessary to either populate lesion sites with tissue-specific regeneration-competent cells or to activate endogenous neural progenitor cells to replace or rescue dying cells.19 Olfactory mucosa contains neurons and sustentacular cells that renew themselves throughout life,1,2 as well as olfactory ensheathing cells that have shown promise in the repair of SCIs.3,4 In human adults, the olfactory mucosa seems to be an excellent source of autologous neuronal precursor cells that can be taken from an easily accessible site.5 These considerations have made the olfactory mucosa an attractive tissue among the several that have been studied for potential applications in axonal regeneration. Inclusion criteria for OMA transplantation in Lima et al.18 were as follows: AIS A or B, age 18 to 40 years, cervical spinal cord lesion <3 cm long or thoracic spinal cord lesion <4 cm, absence of significant nasal and paranasal sinus pathology, and absence of additional serious medical problems, brain disease or psychological disturbance. MEPs were not included among the outcome measures in their trial. The MEP reflects conductivity in the central nervous system, including corticospinal pathways.20 MEPs induced with TMS allow objective assessment of the integrity of the motor circuitry comprising both the corticospinal tract and the peripheral motor nerves.21,22 To our best knowledge, ours is the first case report to demonstrate elicitation of MEPs indicative of electrophysiological conductivity in the human spinal cord after any treatment for chronic, complete SCI. We have previously reported that transplanted olfactory mucosa provides a scaffold for axonal regeneration in a rat model, and that there was transsynaptic and non-transsynaptic neuronal formation in the experimental model. We propose that the outcomes in the present case may be indicative of a similar pathway of neuronal regeneration.9,23,24 Our current clinical trial could provide further evidence to support this hypothesis.
References
Calof AL, Bonnin A, Crocker C, Kawauchi S, Murray RC, Shou J et al. Progenitor cells of the olfactory receptor neuron lineage. Microsc Res Tech 2002; 58: 176–188.
Roisen FJ, Klueber KM, Lu CL, Hatcher LM, Dozier A, Shields CB et al. Adult human olfactory stem cells. Brain Res 2001; 890: 11–22.
Barnett SC, Riddell JS . Olfactory ensheathing cells (OECs) and the treatment of CNS injury: advantages and possible caveats. J Anat 2004; 204: 57–67.
Smith PM, Lakatos A, Barnett SC, Jeffery ND, Franklin RJ . Cryopreserved cells isolated from the adult canine olfactory bulb are capable of extensive remyelination following transplantation into the adult rat CNS. Exp Neurol 2002; 176: 402–406.
Feron F, Perry C, McGrath JJ, Mackay-Sim AF . New techniques for biopsy and culture of human olfactory epithelial neurons. Arch Otolaryngol Head Neck Surg 1998; 124: 861–866.
Moore MJ, Friedman JA, Lewellyn EB, Mantila SM, Krych AJ, Ameenuddin S et al. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials 2006; 27: 419–429.
Stokols S, Tuszynski MH . Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials 2006; 27: 443–451.
Aoki M, Kishima H, Yoshimura K, Ishihara M, Ueno M, Hata K et al. Limited functional recovery in rats with complete spinal cord injury after transplantation of whole-layer olfactory mucosa: laboratory investigation. J Neurosurg Spine 2010; 12: 122–130.
Iwatsuki K, Yoshimine T, Kishima H, Aoki M, Yoshimura K, Ishihara M et al. Transplantation of olfactory mucosa following spinal cord injury promotes recovery in rats. Neuroreport 2008; 19: 1249–1252.
Nakayama J, Takao T, Kiuchi H, Yamamoto K, Fukuhara S, Miyagawa Y et al. Olfactory mucosal transplantation after spinal cord injury improves voiding efficiency by suppressing detrusor-sphincter dyssynergia in rats. J Urol 2010; 184: 775–782.
Lima C, Escada P, Pratas-Vital J, Branco C, Arcangeli CA, Lazzeri G et al. Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil Neural Repair 2009; 24: 10–22.
Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011; 34: 535–546.
Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 1994; 91: 79–92.
Calancie B, Harris W, Brindle GF, Green BA, Landy HJ . Threshold-level repetitive transcranial electrical stimulation for intraoperative monitoring of central motor conduction. J Neurosurg 2001; 95: 161–168.
Cros D, Soto O, Chiappa KH . Transcranial magnetic stimulation during voluntary action: directional facilitation of outputs and relationships to force generation. Brain Res 2007; 1185: 103–116.
Brucker BS, Bulaeva NV . Biofeedback effect on electromyography responses in patients with spinal cord injury. Arch Phys Med Rehabil 1996; 77: 133–137.
Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 2011; 377: 1938–1947.
Lima C, Pratas-Vital J, Escada P, Hasse-Ferreira A, Capucho C, Peduzzi JD et al. Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study. J Spinal Cord Med 2006; 29: 191–206.
Stocum DL . Stem cells in CNS and cardiac regeneration. Adv Biochem Eng Biotechnol 2005; 93: 135–159.
Rossini PM . The anatomic and physiologic bases of motor-evoked potentials. Neurol Clin 1988; 6: 751–769.
Calancie B, Alexeeva N, Broton JG, Suys S, Hall A, Klose KJ . Distribution and latency of muscle responses to transcranial magnetic stimulation of motor cortex after spinal cord injury in humans. J Neurotrauma 1999; 16: 49–67.
Curt A, Keck ME, Dietz V . Functional outcome following spinal cord injury: significance of motor-evoked potentials and ASIA scores. Arch Phys Med Rehabil 1998; 79: 81–86.
Iwatsuk K, Yoshimine T, Sankai Y, Umegaki M, Ohnishi Y, Ishihara M et al. Transplantation of olfactory mucosa as a scaffold for axonal regeneration following spinal cord contusion in rats. Neurosci Med 2013; 4: 112–116.
Moriwaki T, Iwatsuki K, Mochizuki-Oda N, Ohnishi Y, Ishihara M, Umegaki M et al. Presence of trans-synaptic neurons derived from olfactory mucosa transplanted after spinal cord injury. Spine (Phila Pa 1976) 2014; 39: 1267–1273.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Iwatsuki, K., Tajima, F., Sankai, Y. et al. Motor evoked potential and voluntary EMG activity after olfactory mucosal autograft transplantation in a case of chronic, complete spinal cord injury: case report. Spinal Cord Ser Cases 2, 15018 (2016). https://doi.org/10.1038/scsandc.2015.18
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2015.18