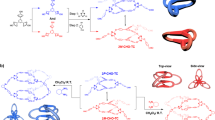
Abstract
Chiral self-sorting is a phenomenon wherein racemic components are spontaneously sorted into homo- or heterochiral molecular assemblies through chiral discrimination between the components. Chiral self-sorting may be related to biological molecular systems where chiral biomolecules are concerned, but the detail of this sorting process has been unclear. Here we show the chiral self-sorting process in the formation of a homochiral Pd2L4 coordination cage from a racemic mixture of a binaphthol-based ditopic ligand by quantitative analysis of self-assembly process (QASAP). The self-assembly of the cage mainly takes place through two pathways that branch off from the intermolecular reaction of mononuclear complexes. Even though the homochiral cages are thermodynamically the most stable, heterochiral intermediates were preferentially produced at first under kinetic control, which were eventually converted into the homochiral cages. Our results reveal complicated pathways in chiral self-sorting.
Similar content being viewed by others
Introduction
Chiral recognition has been thought to be one of the important phenomena relevant to the origin of life, where only one-handed chiral structures, D and L isomers, were selected as constituents of biological structures such as DNAs and proteins1,2,3,4,5,6,7,8. Thus chiral recognition has attracted much attention for the purpose of in-depth understanding of why and how one of the enantiomers was selected and has been amplified in life. Chiral self-sorting is a phenomenon that racemic components are spontaneously sorted into homo- or heterochiral molecular assemblies through chiral discrimination between the components. Although chiral self-sorting is not directly related to the selection of one of the enantiomers in the origin of life, it may be relevant to biological molecular systems where chiral biomolecules are concerned. Encouraged by elaborate biological systems, simplified artificial examples of chiral self-sorting have been reported in molecular self-assembly of racemic components9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43. Biasing toward homochiral or heterochiral assembled structures takes place under thermodynamic control based on the recognition between chiral molecular building blocks. The strategies for narcissistic (homochiral) or social (heterochiral) self-sorting in supramolecular systems are well categorized9,10,11,12,13,14,15,26,44,45,46,47,48,49,50,51,52,53. However, chiral self-sorting process has remained to be seen mainly due to lacking an appropriate analytical method for the quantitative investigation of molecular self-assembly processes.
Recently, we have developed a method for the investigation of molecular self-assembly processes based on the concept that the information about the intermediates transiently produced during molecular self-assembly, most of which cannot be detected by spectroscopy, can indirectly be obtained as the average composition of all the intermediates by the quantification of all the substrates and the products (QASAP: quantitative analysis of self-assembly process)54,55,56,57,58,59,60,61,62,63. QASAP has enabled us to reveal the self-assembly processes of coordination cages55,56, capsules57,58,59, sphere60 and rings61,62,63.
Here we report the chiral self-sorting process of an enantiomeric pair of homochiral cages consisting of two Pd(II) ions and four one-handed BINOL-based ditopic ligands64 along the self-assembly pathways of the cage revealed by QASAP. During the self-assembly of the homochiral cages, more heterochiral dinuclear intermediates than under the statistical ratio are firstly produced by intermolecular reactions of mononuclear species. Then the correction of chirality in the heterochiral cages and the dinuclear intermediates takes place with the aid of free ditopic ligands to lead to the homochiral cages, indicating complicated pathways in the chiral self-sorting.
Results
Self-assembly process of the cage from 1S
Prior to discussing the chiral self-sorting process of the homochiral Pd214 cages (Fig. 1), the self-assembly process of the Pd21S4 homochiral cage from one of the enantiomers of the binaphthol (BINOL)-based ditopic ligand 1S and PdPy*4(BF4)2 in CD3NO2 and CD2Cl2 (4:1, v/v) at 298 K was investigated by QASAP (Eq. (1) and Fig. 2).
where Py* indicates 3-chloropyridine. The self-assembly of the Pd21S4 cage was monitored by 1H NMR spectroscopy (Fig. 3 and Supplementary Figs 1 and 2). Many signals not assigned to either the substrates (1S and PdPy*4(BF4)2) or the products (Pd21S4 and Py*) appeared at 5 min and disappeared within 60 min. These apparent signals for the intermediates were not found after 60 min but 40% of 1S still remained in the intermediates at 60 min (yellow line in Fig. 4a). This result indicates that the 1H-NMR-observable primitive intermediates (IntP) would be converted into the intermediates that cannot be observed by 1H NMR, which finally led to the Pd21S4 cage within 12 h.
Chiral self-sorting process of the homochiral cages. The major pathway of the self-assembly of the homochiral cages is shown. A mixture of PdPy*4(BF4)2 (Py*: 3-chloropyridine) and a racemic mixture of ditopic ligand 1 in CD3NO2 and CD2Cl2 (4:1, v/v) at 298 K affords mononuclear species (IntP), mainly consisting of Pd1Py*3 and Pd12Py*2, without a narcissistic preference of the ligand chirality. Then the intermolecular reaction between IntP leads to dinuclear complexes Pd214Py*3, where the heterochiral species are more preferred than the statistical ratio. Although the amount of the homochiral Pd214Py*3 is small, the faster intramolecular ligand exchanges in the homochiral intermediates lead to the homochiral cages faster. Then the heterochiral cages are produced from the heterochiral intermediates. The conversion of the heterochiral intermediates and cages into the homochiral counterparts takes place in the late stage of the self-assembly
Self-assembly process of the cage(s). As the self-assembly processes of the Pd214 cage(s) from 1S and from a racemic mixture of 1 are the same except diastereomers for the intermediates and the cages, the chirality of 1 (1S or 1R) is not shown in this figure. The dashed arrows indicate minor pathways. IntP indicates the primitive intermediates that exist in the beginning of the self-assembly of the cage (from 5 to 60 min). One of the representative isomers is shown for Pd214Py*3 (A) and Pd213Py*4. Py* indicates 3-chloropyridine
1H NMR spectra of the self-assembly of the cage from 1S. 1H NMR spectra (500 MHz, 298 K) of the Pd21S4(BF4)4 cage and PdPy*4(BF4)2 in CD3NO2 and of 1S and the reaction mixture for the self-assembly of the Pd21S4(BF4)4 cage from 1S ([1S]0 = 1.63 mM) and PdPy*4(BF4)2 ([Pd]0 = 0.815 mM) in CD3NO2 and CD2Cl2 (4:1, v/v) at 298 K. a 4.7–9.4 ppm. The signals coloured in blue, brown, purple and green indicate the Pd21S4(BF4)4 cage, PdPy*4(BF4)2, 1S and Py*, respectively. The signal marked with the black solid circle at 8.51 ppm indicates an impurity in CD3NO2. The signal marked with the black open triangle at 7.52 ppm indicates CHCl3, which was used during the preparation of the reaction mixture (details are shown in Methods). b 2.4–2.7 ppm. Red solid circles indicate Hg signals in IntP
Quantitative analysis of the self-assembly of the cage from 1S. a Existence ratios of the substrates (1S and PdPy*4(BF4)2), the products (Pd21S4(BF4)4 and Py*), IntP (primitive intermediates observed by 1H NMR from 5 to 60 min) and Int (all the intermediates including IntP). b The (〈n〉, 〈k〉) plot for the self-assembly of the Pd21S4(BF4)4 cage from 1S ([1S]0 = 1.6 mM) and PdPy*4(BF4)2 ([Pd]0 = 0.8 mM). A blue line with open circles indicates the change in the (〈n〉, 〈k〉) value. Error bars indicate the standard errors. Red cross hairs indicate the (n, k) value of the species Pd a 1 b Py* c , which is indicated as (a, b, c). The (〈n〉, 〈k〉) value indicates the (n, k) value for the average composition of all the intermediates determined by experiment, \({\mathrm{Pd}}_{\left\langle a \right\rangle }{\mathbf{1}}_{\left\langle b \right\rangle }{\mathrm{Py}}_{\left\langle c \right\rangle }^ \ast\). c The definition of the (n, k) value showing Pd1S2Py*2 as an example
The self-assembly process was then investigated by n–k analysis (Fig. 4b and Supplementary Tables 1–5)54. The intermediates of the self-assembly in Eq. (1) are generally expressed by Pd a 1 b Py* c (a, b and c are positive integer or 0). The (n, k) value for Pd a L b Py* c is defined by Eqs. (2) and (3).
The n value indicates the average number of Pd(II) ions bound to a single ligand L, while the k value represents the ratio between Pd(II) and the ligand (Fig. 4c). The average composition of all the intermediates, \({\mathrm{Pd}}_{\left\langle a \right\rangle }{\mathrm{L}}_{\left\langle b \right\rangle }{\mathrm{Py}}_{\left\langle c \right\rangle }^ \ast\), can be determined from the difference between the consumption ratio and the formation ratio of each component in Eq. (1) (see Methods). The 〈n〉 and 〈k〉 values can be expressed using 〈a〉, 〈b〉 and 〈c〉 as follows:
Therefore, n–k analysis enables us to investigate molecular self-assembly process based on the average composition of all the intermediates even if none of the intermediates can be detected by spectroscopy.
From 5 to 60 min, the 〈k〉 value was almost constant at 0.55, which is higher than 0.5, indicating that slightly more Pd(II) ions were incorporated into the intermediates than in the stoichiometric ratio of the substrates ([Pd]0:[1S]0 = 1:2). The 〈n〉 value increased from 1.1 to 1.9 during this period, which correlates to the release of a large amount of Py*s (Fig. 4a). As the consumption of both the substrates in Eq. (1) continued after 5 min, it is true that intermolecular ligand exchanges of the substrates took place, but a large increase in the 〈n〉 value would be mainly due to intra- and/or intermolecular ligand exchanges in/between the intermediates. From 1 to 3 h, the 〈k〉 value increased with an almost constant value of the 〈n〉 value at 1.87. Considering that both the substrates were slowly consumed with the production of a large amount of the cages during this period, the increase in the 〈k〉 value is mainly due to the release of the cage from a mixture of the intermediates.
The species whose (n, k) value is close to the (〈n〉, 〈k〉) values obtained by experiment were Pd21S3Py* c (c = 2–4) and Pd21S4Py* c (c = 1–3) (red cross hairs in Fig. 4b). Then the self-assembly of the Pd21S4 cage was monitored by ESI-TOF mass spectrometry (Supplementary Figs 8a, 9a, 10a and 11a and Supplementary Table 17). The signal for Pd21S3Py*2 was observed (m/z = 542.11 for [Pd21S3Py*2]4+). As to Pd21S4Py* c (c = 1–3), on the other hand, only the signals for the Pd21S4 cage were detected (m/z = 629.67 for [Pd21S4]4+, 868.55 for [Pd21S4·(BF4)]3+ and 1346.32 for [Pd21S4·(BF4)2]2+). The coordination ability of Py* is weaker than that of the pyridyl groups in 1, and Py* tends to leave the Pd(II) centres during the ionization process58. This tendency was confirmed by the observation of Pd1SPy*2, whose Pd(II) centre is coordinately unsaturated. A part of the observed signal should thus be derived from the species that have more Py* than those observed by ESI-TOF mass spectrometry, and Pd21S4Py* c (c = 1–3) should exist in the reaction mixture.
Considering that n–k analysis and the ESI-TOF mass monitor of the self-assembly suggest that dinuclear complexes were already produced in the beginning of the self-assembly, IntP, which are considered to be precursory species of the dinuclear intermediates, should be mononuclear complexes. The existence ratio of IntP at 5 min based on 1S is 48%, while that of all the intermediates is 70% (Fig. 4a and Supplementary Table 1), indicating that 22% of 1S should be incorporated in the dinuclear intermediates that could not be detected by 1H NMR spectroscopy. IntP was analysed with the 1H NMR spectrum measured at 5 min, in which eight chemically inequivalent Hg signals were observed (Fig. 3b and Supplementary Fig. 2). This result suggests that IntP contains a mixture of mononuclear complexes possessing different number of 1S, Pd1S b Py*4–b (b = 1–4). The 〈k〉 value of about 0.55 until 1 h (Fig. 4b) indicates that the ratio of Pd and 1S of the average composition of IntP is [Pd]:[1S] = 1:1.82, so main species of IntP should be Pd1SPy*3 and Pd1S2Py*2. According to the symmetry of Pd1S b Py*4–b (b = 1–3) (Fig. 5a), the total number of chemically inequivalent methyl groups of 1S in Pd1SPy*3 and cis- and trans-Pd1S2Py*2 is 8, which is the same as the number of signals observed in the 1H NMR spectra. The (〈n〉, 〈k〉) value at 5 min (1.13, 0.56) is far from that for Pd1SPy*3 (1.00, 1.00) but is close to that for Pd1S2Py*2 (1.00, 0.50), suggesting that more Pd1S2Py*2 existed than Pd1SPy*3 at 5 min. In the following discussion, Pd1SPy*3 and cis and trans isomers of Pd1S2Py*2 are considered to be the main species in IntP.
Symmetry of mononuclear and dinuclear intermediates. a Symmetry of mononuclear species. Blue, red and green arrows indicate 1S, 1R and Py*, respectively. Yellow circles indicate Pd(II) ions. Labels a–f indicate chemically inequivalent methyl groups of 1 (Hg). b Symmetry of heterochiral cages. Blue and red arrows indicate 1S and 1R, respectively. Yellow circles indicate Pd(II) ions. Labels a–d indicate chemically inequivalent methyl groups in 1 (Hg). One Pd(II) ion is eclipsed by the front Pd(II) ion
After 5 min, intermolecular ligand exchanges between IntPs produce dinuclear complexes, Pd21S3Py*4 and Pd21S4Py*3 (Eqs. (6) and (7)).
According to the 〈k〉 value of 0.55 (Fig. 4b), it is suggested that Pd21S4Py*3 (k = 0.5) is more preferentially formed than Pd21S3Py*4 (k = 0.67), which is consistent with the fewer amount of Pd1SPy*3 in IntP. Then intramolecular ligand exchanges in Pd21S3Py*4 and in Pd21S4Py*3 take place with the release of Py*s, which causes the increase in the 〈n〉 value with time (Fig. 4b). It is worth noting that the isomer of Pd21S4Py*3 (B in Fig. 2) derived from the reaction of cis- and trans-Pd1S2Py*2 cannot lead to the cage by simple intramolecular ligand exchanges (Fig. 2), and thus this Pd21S4Py*3 isomer (B) would finally be converted into the Pd21S3Py*2 partial cage with the release of Py* and 1S. The successive intramolecular ligand exchanges in the other isomers of Pd21S4Py*3 (A in Fig. 2) finally leads to the Pd21S4 cage (the product). Pd21S3Py*4 produced by Eq. (6) is converted into the Pd21S3Py*2 partial cage and its further transformation toward the cage requires the incorporation of a ditopic ligand (free 1S or IntP) through an intermolecular ligand exchange, which should be slower than the intramolecular ligand exchanges. As a consequence, the amount of Pd21S4Py* c (c = 1–3) in the intermediates should decrease faster than that of Pd21S3Py* c (c = 2–4). Thus the 〈k〉 value increased after 60 min as a result of the increase in the relative ratio of Pd21S3Py* c (c = 2 and 3) in the intermediates. The self-assembly process of the Pd21S4 cage from 1S and PdPy*4(BF4)2 is summarized in Fig. 2.
Self-assembly process of the cages from 1S and 1R
Next, the formation of the homochiral cages from a racemic mixture of 1S and 1R in the same condition (CD3NO2 and CD2Cl2 (4:1, v/v) at 298 K) was investigated by QASAP. The rate of the formation of the homochiral cages (Pd21S4 and Pd21R4) (blue solid circles in Fig. 6) is significantly slower than that of Pd21S4 from 1S (blue open circles in Fig. 6) but the rates of the release of Py* in both the cases are similar to each other (green solid and open circles). This result suggests that heterochiral species containing 1S and 1R should mistakenly (kinetically) be produced during the self-assembly. Indeed, the 1H NMR signals for the heterochiral cages were observed, which will be discussed later.
Comparison of the self-assembly from 1S or a racemic mixture of 1S and 1R. Existence ratios of the homochiral cages (Pd21S4 (blue open circles) or a racemic mixture of Pd21S4 and Pd21R4 (blue solid circles)) and Py* for the self-assembly of the homochiral cages from 1S (or a racemic mixture of 1S and 1R) and PdPy*4(BF4)2 in CD3NO2 and CD2Cl2 (4:1, v/v) at 298 K. Green open and solid circles indicate the existence ratio of Py* starting from 1S or a racemic mixture of 1S and 1R, respectively
The 1H NMR spectrum for the self-assembly of the homochiral cages from a racemic mixture of 1S and 1R at 5 min was very similar to that for the self-assembly from 1S at 5 min (Fig. 7 and Supplementary Figs 3 and 4), which suggests the formation of similar mononuclear Pd(II) complexes (IntP) in the very early stage of the self-assembly. The number of Hg signal observed in Fig. 7b is more than that in Fig. 3b, which indicates the formation of heterochiral Pd12Py*2 (Fig. 5a) as IntP. After 5 min, these signals disappeared and five new signals that were not observed in the case of the self-assembly from 1S appeared at 30 min and increased until 3 h. These chemically inequivalent Hg signals were observed in 2.5–2.6 ppm (Fig. 7b and Supplementary Fig. 4). 1H DOSY measurement of the reaction mixture at 3 h (Supplementary Fig. 5 and Supplementary Table 16) indicates that the diffusion coefficients of these signals are similar to that for the homochiral cages, suggesting that the species mainly produced from 30 min to 3 h are a mixture of heterochiral cages (Fig. 1). According to the symmetry of the heterochiral cages (Pd21S31R (or Pd21S1R3) and cis- and trans-Pd21S21R2) shown in Fig. 5b, the total number of chemically inequivalent methyl groups of 1 is 7, suggesting that some of the Hg signals should overlap with each other. The change in the integrals of the five H1–H5 signals with time was analysed (Supplementary Figs 4, 6 and 7) and it was found that the integral ratios of H1, H3 and H5 were kept to be 1:2:1 and that the integrals of H2 and H4 changed independently. As to Pd21S31R in Fig. 5b, the environments of a and b are similar. In the same way, the environments of c and d in Pd21S31R are similar, so two of the four chemically inequivalent Hg signals of Pd21S31R (and its enantiomer, Pd21S1R3) would overlap with each other to show the three signals of H1, H3 and H5 in a 1:2:1 ratio. cis-Pd21S21R2 has two chemically inequivalent methyl groups, while all the methyl groups in trans-Pd21S21R2 are chemically equivalent (Fig. 5b), so two of the three Hg signals for cis- and trans-Pd21S21R2 would overlap with each other. The intensity of H4 is about three times as large as that of H2 at 1 h and decreased with time and became similar to that of H2 at 6 h (Supplementary Fig. 6). Thus, it is the best reasonable that H2 is derived from the methyl groups a in cis-Pd21S21R2, while H4 is from the methyl groups b in cis-Pd21S21R2 and a in trans-Pd21S21R2, considering the similar environment of the methyl groups b in cis-Pd21S21R2 and a in trans-Pd21S21R2. The faster decrease in the H4 signal than that in H2 due to the faster conversion of trans-Pd21S21R2 is consistent with the less stability of trans-Pd21S21R2 than the cis isomer (Supplementary Fig. 13 and Supplementary Table 20). As will be described below, a discussion on the chiral self-sorting process is possible not depending on the assignment of each heterochiral cage. After 3 h, the signals for the heterochiral cages decreased, which was concomitant with the increase of the signals for the homochiral cages.
1H NMR spectra of the self-assembly of the cages from a racemic mixture of 1S and 1R. 1H NMR spectra (500 MHz, 298 K) of the Pd21S4(BF4)4 and Pd21R4(BF4)4 cages and PdPy*4(BF4)2 in CD3NO2 and of a racemic mixture of 1S and 1R and the reaction mixture for the self-assembly of the homochiral cages (Pd21S4(BF4)4 and Pd21R4(BF4)4) from a racemic mixture of 1S and 1R ([1S + 1R]0 = 1.65 mM) and PdPy*4(BF4)2 ([Pd]0 = 0.825 mM) in CD3NO2 and CD2Cl2 (4:1, v/v) at 298 K. a 4.7–9.4 ppm. The signals coloured in blue, brown, purple and green indicate the homochiral cages, PdPy*4(BF4)2, 1 (a racemic mixture of 1S and 1R) and Py*, respectively. The signal marked with the black solid circle at 8.51 ppm indicates an impurity in CD3NO2. The signal marked with the black open triangle at 7.52 ppm indicates CHCl3, which was used during the preparation of the reaction mixture (details are shown in Methods). b 2.4–2.7 ppm. Red solid circles indicate Hg signals in IntP. Signals with a number (1–5) indicate the Hg signals of the heterochiral cages. H1, H3 and H5 are assigned to Pd21S1R3 (and its enantiomer, Pd21S31R). H2 and H4 signals are assigned to one of the Hg signals of cis-Pd21S21R2 and to the other Hg signal of cis-Pd21S21R2 and the Hg signal of trans-Pd21S21R2 (overlapped), respectively
ESI-TOF mass spectrometry of the reaction mixture from a racemic mixture of 1S and 1R showed the same species as was detected in the self-assembly of the Pd21S4 cage from 1S (Pd1Py*2, Pd213Py*2 and Pd214) (Supplementary Figs. 8b, 9b, 10b and 11b and Supplementary Table 18), suggesting that the self-assembly processes of the homochiral cage(s) from 1S and from a mixture of 1S and 1R are the same as long as the formation of diastereomeric isomers is ignored. A similarity in the self-assembly process between from 1S and from a racemic mixture of 1S and 1R was also confirmed by n–k analysis (the heterochiral cages are not included in the intermediates in Fig. 8b). The 〈n〉 value increased with an almost constant 〈k〉 value from 5 to 60 min. After 1 h, the 〈k〉 value increased (Fig. 8b), mainly because more Pd(II) ions were incorporated into the intermediates than free ligands (brown line in Fig. 8a).
Quantitative analysis of the self-assembly of the cages from a racemic mixture of 1S and 1R. a Existence ratios of the substrates (a racemic mixture of 1S and 1R and PdPy*4(BF4)2), the products (Pd21S4(BF4)4, Pd21R4(BF4)4 and Py*) and Int (all the intermediates excluding the heterochiral cages). b A blue line with open circles indicates the change in the (〈n〉, 〈k〉) value for the self-assembly of the homochiral cages from a racemic mixture of 1S and 1R ([1S + 1R]0 = ca. 1.6 mM) and PdPy*4(BF4)2 ([Pd]0 = ca. 0.8 mM). Error bars indicate the standard errors. Red cross hairs indicate the (n, k) value of the species Pd a 1 b Py* c , which is indicated as (a, b, c)
It is worth noting that free ditopic ligands remained in the reaction mixture during the self-assembly (purple line in Fig. 8a). Considering the associative ligand exchange mechanism on Pd(II) centres65, the heterochiral cages cannot be converted into the homochiral ones without breaking the Pd–N bonds in the heterochiral cages initiated by the coordination of a free ligand to the Pd(II) centre. Such a role of the free ligand for the conversion of kinetically produced species into thermodynamically the most stable structure has been observed in other Pd(II)-based self-assemblies56,59,60.
Chiral self-sorting process of the homochiral cages
It was found that in the self-assembly of the homochiral cages from a racemic mixture of 1S and 1R mononuclear species (IntP, which mainly contains Pd1Py*3 and Pd12Py*2) are initially produced and then converted into (homo- and heterochiral) Pd213Py* c (c = 2–4) and Pd214Py* c (c = 1–3), which are transformed into a mixture of homo- and heterochiral cages, and that the cage formation through Pd214Py* c (c = 1–3) is the main pathway. In order to discriminate homo- and heterochiral species by ESI-TOF mass spectrometry, the self-assembly of the cage from a 1:1 mixture of 1S and partially deuterated 1R (1R-d6) was carried out under the same condition (Fig. 1). In this experiment, the species that contain the different number of enantiomeric ditopic ligands can clearly be distinguished but the stereoisomers cannot.
To investigate the chiral self-sorting process in the dominant pathway of the cage formation, the time development of the signals for [Pd1Py*2]2+ and [Pd214]4+ was monitored by ESI-TOF mass spectrometry (Supplementary Figs 9b, 10b and 11b)66,67. As to [Pd1Py*2]2+, the signals for the two species, [Pd1SPy*2]2+ and [Pd(1R-d6)Py*2]2+, with the same intensity decreased simultaneously (Supplementary Fig. 9b), indicating that the conversion rates of chiral isomers of IntP are almost the same. As mentioned above, the signal for [Pd214]4+ is derived from the species Pd214Py* c (c = 0–3). The signals for all the five chiral isomers, [Pd21S4]4+ (S,S,S,S),[Pd21S3(1R-d6)]4+ (S,S,S,R),[Pd21S2(1R-d6)2]4+(S,S,R,R), [Pd21S(1R-d6)3]4+ (S,R,R,R) and [Pd2(1R-d6)4]4+ (R,R,R,R) were detected (Supplementary Fig 11b) and their relative existence ratio was determined under the assumption that the five diastereomers are ionized with the same probability (Supplementary Fig. 12 and Supplementary Table 19). The ratio for (S,S,S,S), (S,S,S,R), (S,S,R,R), (S,R,R,R) and (R,R,R,R) at 15 min was 1:5.4:10.2:5.4:1, which indicates that the heterochiral species ((S,S,S,R), (S,S,R,R) and (S,R,R,R)) are more favoured than the statistical ratio (1:4:6:4:1). This result indicates that chiral self-sorting already took place towards the heterochirality to some extent in the formation of the dinuclear intermediates. Then the signals for the homochiral species, (S,S,S,S) and (R,R,R,R), were intensified with the decrease in the signals for the heterochiral species (Supplementary Fig. 11b). As far as we know, this is the first report on the monitor of chiral self-sorting process.
To quantitatively discuss the degree of chiral self-sorting, we defined parameter X, which expresses how much a system is biased towards homo- or heterochiral assemblies. Now we consider a general case where the two enantiomeric homochiral isomers and m of heterochiral isomers (homo, hetero1, hetero2, ······, heterom–1, heterom and homo) are produced. When the relative ratio for the species observed by experiment is expressed by 1:a1:a2: ······:am–1:a m :1 and in the same way the statistical ratio of the species is indicated by 1:s1:s2: ······:sm–1:s m :1, the parameter X is defined by Eq. (8).
When the diastereomeric isomers are distributed under the statistical ratio (no preference), X = 0. When X is positive, homochiral isomers are favoured and X = 1 indicates the perfect homochiral self-sorting (no heterochiral species). When X is negative, heterochiral isomers are preferentially produced.
For the self-assembly of the Pd214 cage from a racemic mixture of 1S and 1R, the X value was determined from the NMR signals for the homo- and heterochiral cages and from the mass signals for [Pd214]4+, separately, so the X values determined from the two experimental data are indicated as XNMR and XMS, respectively. As XNMR can be determined by the total amount of the heterochiral cages (the integrals of the H1–H5 signals) and the amount of the homochiral cages (the integral of the signal at 2.46 ppm), the ambiguity of the assignment of the Hg signals for the isomers of the heterochiral cages (Pd21S31R and cis- and trans-Pd21S21R2) does not affect the XNMR value at all. The XMS and XNMR values provide valuable information on the chiral self-sorting process. The XNMR value (a blue line in Fig. 9) indicates the distributions of the homo- and heterochiral cages. The positive XNMR value throughout the self-assembly indicates that thermodynamically the most stable homochiral cages were preferentially produced. However, the change in XNMR was not simple. XNMR was 0.71 at 15 min, then decreased until 30 min and increased after 30 min to reach 1.0 in the end of the self-assembly. In order to discuss this strange change in XNMR, the change in the XMS value was analysed.
Time variation of the XNMR and XMS values. X indicates the degree of the homo- or heterochiral self-sorting. The definition of the parameter X is indicated in Eq. (8). Blue and red open circles indicate the X values determined based on NMR (XNMR) and ESI-TOF mass (XMS) measurements, respectively. a Plots of XNMR and XMS from 15 min to 3 h. b Plots of XNMR and XMS from 15 min to 3 days
The XMS value (red open circles in Fig. 9 and Supplementary Table 19) is based on the mass signals for [Pd214]4+, which are derived from Pd214Py* c (c = 0–3). Thus the XMS value contains the information about the intermediates, Pd214Py* c (c = 1–3), besides the cages. The negative value of XMS at 15 min (–0.50) indicates that heterochiral species are favoured. Considering the fact that the homochiral cages were preferentially produced at 15 min, the intermediates are biased towards heterochirality. Thus the reaction mixture at 15 min predominantly contains the homochiral cages and the heterochiral intermediates (as Fig. 8a shows, the substrates and Py* are also included). This indicates that though the amount of homochiral intermediates was significantly low, the homochiral cages were exclusively produced, suggesting that the intramolecular ligand exchanges in the homochiral intermediates, Pd21S4Py* c and Pd21R4Py* c (c = 1–3), took place much faster than in the heterochiral intermediates. The decrease in the XNMR value after 15 min suggests that most of the homochiral intermediates were converted into the homochiral cages and that the heterochiral cages began to be produced from the heterochiral intermediates. The increase in the XNMR value after 30 min indicates that the conversion of the heterochiral species into the homochiral ones took place.
The XMS value gradually increased with time but was smaller than the XNMR value until 12 h. This indicates that the chirality of the intermediates is still more biased towards heterochirality than that of the cage. The XMS value increased as high as the XNMR value at 12 h. Because the intermediates (about 20% based on 1) remained in the reaction mixture at 12 h (Fig. 8a), the chirality of the intermediates was as much biased towards homochirality as that of the cage from 3 to 12 h, which suggests that the correction of the chirality (from hetero to homo) in the intermediates also took place during this period. The change in the XMS value indicates that heterochiral species are favoured under kinetic control even though the homochiral cages are thermodynamically the most favoured.
Discussion
The chiral self-sorting process was investigated for the self-assembly of the homochiral Pd214 cages consisting of BINOL-based ditopic ligands. The self-assembly processes of the cage(s) from 1S and from a racemic mixture of 1S and 1R were analysed by QASAP. At first, mononuclear species (IntP), which are mainly Pd1Py*3 and Pd12Py*2, are produced. Then the intermolecular reactions between IntPs lead to Pd213Py*4 and Pd214Py*3, in which intramolecular ligand exchanges take place to lead to the Pd213Py*2 partial cage and the Pd214 cage. Finally, Pd213Py*2 is converted into the cage by the incorporation of 1 (Fig. 2). According to the (〈n〉, 〈k〉) value, Pd214Py* c (c = 1–3) are the dominant dinuclear intermediates (major pathway). The chiral self-sorting process along the dominant self-assembly pathway takes place in a complicated manner (Fig. 1). The heterochiral Pd214Py* c (c = 1–3) are preferentially produced at first but the faster intramolecular ligand exchanges in the homochiral Pd214Py* c (c = 1–3) lead to the homochiral cages faster. Then the heterochiral cages are produced from the heterochiral intermediates. The conversion of the heterochiral intermediates and cages into the homochiral counterparts takes place in the late stage of the self-assembly. Based on the self-assembly process of the cage, the kinetic distribution of the diastereomeric isomers must be destined in the stage of the intermolecular reactions between IntPs. These results indicate that the chiral self-sorting process is not simple and that homochiral intermediates are not necessarily preferred under kinetic control even though the homochiral final products are thermodynamically the most stable.
Methods
General information
1H and 13C NMR spectra were recorded using a Bruker AV-500 (500 MHz) spectrometer. All 1H spectra were referenced using a residual solvent peak, CD3NO2 (δ 4.33), CDCl3 (δ 7.26) and DMSO-d6 (δ 2.50). All 13C NMR spectra were referenced using a residual solvent peak, CDCl3 (δ 77.16) and DMSO-d6 (δ 39.52). Electrospray ionization time-of-flight (ESI-TOF) mass spectra were obtained using a Waters Xevo G2-S Tof mass spectrometer.
Materials
Unless otherwise noted, all solvents and reagents were obtained from commercial suppliers (TCI Co., Ltd., WAKO Pure Chemical Industries Ltd., KANTO Chemical Co., Inc., and Sigma-Aldrich Co.) and were used as received. CD3NO2 was purchased from Acros Organics and used after dehydration with Molecular Sieves 4 Å. Ditopic ligand 1S, 1R and a racemic mixture of 1S and 1R were prepared according to the literature64. PdPy*4(BF4)2 was synthesized according to the literature61.
Synthesis of 1R-d 6
A synthetic scheme of 1R-d6 is shown in Supplementary Fig. 14.
Preparation of dimethoxymethane-d668: A solution of paraformaldehyde (3.00 g, 100 mmol) and p-toluenesulfonic acid (172 mg, 1.0 mmol) in CD3OD (10 mL) was stirred at 50 °C for 16 h under nitrogen atmosphere. The obtained solution was distilled to afford dimethoxymethane-d6 (5.64 g, 74%). 1H NMR (500 MHz, CDCl3, 298 K): δ 7.26 (s, 2H); 13C{1H} NMR (125 MHz, CDCl3, 298 K): δ 97.50 (s), 54.32 (sept, JCD = 21.7 Hz); bp: 42 °C (lit. 42 °C for nondeuterated dimethoxymethane).
Preparation of a solution of chloromethyl methyl ether-d3 (as a mixture of ZnBr2 and methyl acetate-d3 in toluene)69: Dimethoxymethane-d6 (500 μL, 5.23 mmol) and acetyl chloride (373 μL, 5.23 mmol) were added to a solution of ZnBr2 (1.18 mg, 5.23 μmol) in toluene (1.5 mL) at room temperature. The reaction mixture was stirred at room temperature for 3 h. The concentration of the obtained solution was determined through the comparison of the signal intensity with [2.2]paracyclophane by 1H NMR (2.06 M). This colourless solution was used without purification. 1H NMR (500 MHz, CDCl3, 298 K): δ 5.47 (s, 2 H).
Preparation of (R)-2,2′-dihydroxy-3,3′-bis(3-pyridylethynyl)-1,1′-binaphthlyl (2R)43: A solution of 1R (40.0 mg, 69.4 μmol) and conc. aq. HCl (1.2 mL, 14 mmol) in CH2Cl2 (2.5 mL) was vigorously stirred at room temperature for 3 h and then quenched by aq. NaOH (2.5 M, 5.6 mL). After addition of CH2Cl2 (10 mL), the organic layer was separated. The aqueous layer was washed with CH2Cl2 (10 mL × 3) and the combined organic layer was dried over anhydrous MgSO4 and filtered. The solvent was removed in vacuo to afford 2R as a colourless solid (23.4 mg, 69%). 1H NMR (500 MHz, DMSO-d6, 298 K): δ 9.02 (s, 2 H), 8.82 (d, JHH = 1.5 Hz, 2 H), 8.60 (dd, JHH = 5.0, 1.4 Hz, 2 H), 8.30 (s, 2 H), 8.04 (dt, JHH = 7.8, 1.6 Hz, 2 H), 7.95 (d, JHH = 7.9 Hz, 2 H), 7.49 (ddd, JHH = 7.9, 4.9, 0.8 Hz, 2 H), 7.34 (ddd, JHH = 8.0, 6.8, 1.2 Hz, 2 H), 7.28 (ddd, JHH = 8.3, 7.0, 1.4 Hz, 2 H), 6.91 (d, JHH = 8.5 Hz, 2 H); 13C{1H} NMR (125 MHz, DMSO-d6, 298 K): δ 152.58, 151.66, 148.88, 138.56, 134.11, 133.67, 128.10, 127.96, 127.52, 124.11, 123.63, 123.54, 119.86, 115.40, 112.72, 90.34, 89.75; HRMS (ESI-TOF) m/z: [M+H]+ calcd. for [C34H21N2O2]+ 489.1598; found 489.1621 and [M+2H]2+ calcd. for [C34H22N2O2]2+ 245.0835; found 245.0846.
Synthesis of 1R-d6: A solution of 2R (20.0 mg, 40.9 μmol) in dry THF (400 μL) and dry DMF (200 μL) was added to a solution of NaH (3.60 mg, 90.0 μmol) in dry DMF (350 μL) at room temperature. The reaction mixture was stirred at room temperature for 1 h. After addition of the solution of chloromethyl methyl ether-d3 in toluene (2.06 M, 70 μL), the reaction mixture was stirred at room temperature for 2 h and quenched by sat. aq. EDTA (2 mL). After the addition of ethyl acetate (2 mL), the organic layer was separated. The organic layer was washed with water (2 mL × 3), dried over anhydrous MgSO4 and filtered. The solvent of the filtrate was removed in vacuo and purified by silica gel column chromatography (silica gel 60 0.040–0.063 mm, AcOEt/Hexane = 2/1) to afford 1R-d6 as a colourless solid (12.8 mg, 54%). 1H NMR (500 MHz, CDCl3, 298 K): δ 8.80 (dd, J = 2.2, 0.9 Hz, 2 H), 8.55 (dd, J = 4.9, 1.6 Hz, 2 H), 8.26 (s, 2 H), 7.87 (d, J = 8.3 Hz, 2 H), 7.83 (ddd, J = 8.0, 2.3, 1.9 Hz, 2 H), 7.45 (ddd, J = 8.1, 6.7, 1.1 Hz, 2 H), 7.32 (ddd, J = 8.3, 6.9, 1.3 Hz, 2 H), 7.29 (ddd, J = 7.8, 4.9, 0.9 Hz, 2 H), 7.24 (d, J = 1.0 Hz, 2 H), 5.14 (d, J = 6.1 Hz, 2 H), 4.94 (d, J = 6.1 Hz, 2 H); 13C{1H} NMR (125 MHz, CDCl3, 298 K): δ 153.15, 152.33, 148.94, 138.51, 134.74, 134.13, 130.43, 127.87, 127.76, 126.68, 126.05, 125.91, 123.28, 120.53, 116.79, 99.13, 90.33, 89.91 (The signal of CD3 around 56 ppm cannot be observed because of C–D coupling.); HRMS (ESI-TOF) m/z: [M+H]+ calcd. for [C38H23D6N2O4]+ 583.2498; found 583.2497 and [M+2H]2+ calcd. for [C38H24D6N2O4]2+ 292.1286; found 292.1295.
Monitoring the self-assembly of Pd214 cage(s) by 1H NMR
A 6.0 mM solution of 1,3,5-trimethoxybenzene in CD3NO2, which was used as an internal standard, was prepared (solution S). A solution of PdPy*4(BF4)2 (12 mM) in CD3NO2 was prepared (solution A). Solution S (50 μL), A (50 μL) and CD3NO2 (400 μL) were added to NMR tube I. The exact concentration of PdPy*4(BF4)2 in solution A was determined through the comparison of the signal intensity with 1,3,5-trimethoxybenzene by 1H NMR. A solution of ditopic ligand 1S or a racemic mixture of 1S and 1R (12 mM) in CHCl3 (100 μL) was added to tube II and the solvent was removed in vacuo. Then the solution S (50 μL), CD2Cl2 (120 μL), and CD3NO2 (380 μL) were added to NMR tube II and the exact amount of 1 in tube II was determined through the comparison of the signal intensity with 1,3,5-trimethoxybenzene by 1H NMR. 0.50 eq. (against the amount of ligand 1 in tube II) of PdPy*4(BF4)2 in solution A (ca. 50 μL; the exact amount was determined based on the exact concentration of solution A and of the ligand in tube II) were added to tube II at 263 K. The self-assembly of the Pd214(BF4)4 cage(s) was monitored at 298 K by 1H NMR spectroscopy. Some of the 1H NMR spectra are shown in Fig. 3 and Supplementary Figs 1 and 2 for the self-assembly from 1S and in Fig. 7 and Supplementary Figs 3 and 4 for the self-assembly from a racemic mixture of 1S and 1R. The exact ratio of 1 and PdPy*4(BF4)2 was unambiguously determined by the comparison of the integral value of each 1H signal of 1,3,5-trimethoxybenzene. The amounts of 1, [PdPy*4]2+, the Pd214(BF4)4 cage(s), IntP (for the self-assembly from 1S) and Py* were quantified by the integral value of each 1H NMR signal against the signal of the internal standard (1,3,5-trimethoxybenzene). In order to confirm the reproducibility, the same experiment was carried out three times (runs 1–3 for the self-assembly from 1S and runs 4–6 for the self-assembly from a racemic mixture of 1S and 1R). The data, the average values of the existence ratios and the (〈n〉, 〈k〉) values are listed in Supplementary Tables 1–5 for the self-assembly from 1S and in Supplementary Tables 6–15 for the self-assembly from a racemic mixture of 1S and 1R, respectively.
The change in the integral values of H1–H5 assigned to the methyl groups of the heterochiral cages is shown in Supplementary Fig. 6. The existence ratios of all the cages (homochiral Pd21S4 and Pd21R4, cis- and trans-Pd21S21R2 and Pd21S11R3 (and Pd21S31R1)) for the self-assembly from PdPy*4(BF4)2 and a racemic mixture of 1S and 1R are shown in Supplementary Fig. 7.
Determination of the existence ratio of each species
The relative integral value of each 1H NMR signal against the internal standard 1,3,5-trimethoxybenzene is used as the integral value in this description. We define the integral values of the signal for the substrates and the products at each time t as follows:
IL(t): 1/6 of the integral value of the g proton in free ligand 1
IM(t): the integral value of the n proton of Py* in [PdPy*4]2+
Icage(t): 1/6 of the integral value of the g proton in the homoleptic Pd214 cage
IPy*(t): the integral value of the n proton of free Py*
I Int P(t): 1/6 of the integral value of the g proton in IntP (for the self-assembly from 1S)
Ih-cage1(t): 1/6 of the integral value of the g proton in the Pd21S11R3 and Pd21S31R1 cages (for the self-assembly from a racemic mixture of 1S and 1R)
Ih-cage2(t): 1/6 of the integral value of the g proton in the cis-Pd21S21R2 cage (for the self-assembly from a racemic mixture of 1S and 1R)
Ih-cage3(t): 1/6 of the integral value of the g proton in the trans-Pd21S21R2 cage (for the self-assembly from a racemic mixture of 1S and 1R)
IM(0) was determined based on the exact concentration of solution A determined by 1H NMR and the exact volume of solution A added into tube II.
IL(0) was determined by 1H NMR measurement before the addition of solution A into tube II.
Existence ratio of [PdPy*4]2+: As the total amount of [PdPy*4]2+ corresponds to IM(0), the existence ratio of [PdPy*4]2+ at t is expressed by IM(t)/IM(0).
Existence ratio of 1: As the total amount of free ligand 1 corresponds to IL(0), the existence ratio of 1 at t is expressed by IL(t)/IL(0).
Existence ratio of Py*: As the total amount of Py* corresponds to IM(0), the existence ratio of Py* at t is expressed by IPy*(t)/IM(0).
Existence ratio of the Pd214 homochiral cage: As the Pd214 homochiral cage is quantified based on 1, the existence ratio of the cage at t is expressed by Icage(t)/IL(0).
Existence ratio of IntP (for the self-assembly from 1S): As IntP is quantified based on 1, the existence ratio of the cage at t is expressed by \({{I}}_{{\mathbf{Int}}^{\mathbf{P}}}\)/IL(0).
Existence ratio of the Pd21S11R3 and Pd21S31R1 cages (for the self-assembly from a racemic mixture of 1S and 1R): As the Pd21S11R3 and Pd21S31R1 cages is quantified based on 1, the existence ratio of the cage at t is expressed by Ih-cage1(t)/IL(0).
Existence ratio of the cis-Pd21S21R2 cage (for the self-assembly from a racemic mixture of 1S and 1R): As the cis-Pd21S21R2 cage is quantified based on 1, the existence ratio of the cage at t is expressed by Ih-cage2(t)/IL(0).
Existence ratio of the trans-Pd21S21R2 cage (for the self-assembly from a racemic mixture of 1S and 1R): As the trans-Pd21S21R2 cage is quantified based on 1, the existence ratio of the cage at t is expressed by Ih-cage3(t)/IL(0).
Existence ratio of all the intermediates (Int): The existence ratio of the total intermediates is determined based on the amount of ligand 1 in the intermediates. Thus the existence ratio of the total intermediate is calculated by subtracting the other species containing 1 (free 1 and the Pd214 cage(s)) from the total amount of 1 (IL(0)). The existence ratio of the total intermediates at t is expressed by (IL(0) – IL(t) – Icage(t))/IL(0) (for the self-assembly from 1S) or (IL(0) – IL(t) – Icage(t) – Ih-cage1(t) – Ih-cage2(t) – Ih-cage3(t))/IL(0) (for the self-assembly from a racemic mixture of 1S and 1R).
〈a〉: The total amount of Pd(II) ions corresponds to IM(0)/4. The amount of Pd(II) ions in [PdPy*4]2+ at t corresponds to IM(t)/4. The amount of Pd(II) ions in the Pd214 cage(s) at t corresponds to Icage(t)/2. The amount of Pd(II) ions in the intermediates at t is thus expressed by IM(0)/4 – IM(t)/4 – Icage(t)/2 (for the self-assembly from 1S) or IM(0)/4 – IM(t)/4 – Icage(t)/2 – Ih-cage1(t)/2 – Ih-cage2(t)/2 – Ih-cage3(t)/2 (for the self-assembly from a racemic mixture of 1S and 1R).
〈b〉: The total amount of ligand 1 corresponds to IL(0). The amount of free ligand 1 at t corresponds to IL(t). The amounts of ligand 1 in the Pd214 cage(s) at t corresponds to Icage(t). The amount of ligand 1 in the intermediates at t is thus expressed by IL(0) – IL(t) – Icage(t) (for the self-assembly from 1S) or IL(0) – IL(t) – Icage(t) – Ih-cage1(t) – Ih-cage2(t) – Ih-cage3(t) (for the self-assembly from a racemic mixture of 1S and 1R).
〈c〉: The total amount of Py* corresponds to IM(0). The amount of free Py* at t corresponds to IPy*(t). The amount of Py* in [PdPy*4]2+ at t corresponds to IM(t). The amount of Py* in the intermediates at t is thus expressed by IM(0) – IPy*(t) – IM(t).
The 〈n〉 and 〈k〉 values are determined with these 〈a〉, 〈b〉 and 〈c〉 values by Eqs. (4) and (5).
Quantitative analysis of self-assembly process
In QASAP, all the substrates (1 and PdPy*4(BF4)2) and the products (Pd214(BF4)4 cage and Py*) were quantified by 1H NMR spectroscopy during the self-assembly of the cage and then the amount of the intermediates not observed by 1H NMR (Int) and the average composition of the unobservable intermediates, \({\mathrm{Pd}}_{\left\langle a \right\rangle }{\bf{1}}_{\left\langle b \right\rangle }{\mathrm{Py}}_{\left\langle c \right\rangle }^ \ast\), were obtained. The existence ratios of the substrates, the products, Int, the heterochiral cages and 〈a〉, 〈b〉, 〈c〉, 〈n〉 and 〈k〉 with time are listed in Supplementary Tables 1–15 and plotted in Figs. 4 and 8.
1H DOSY NMR spectroscopy
1H DOSY NMR spectra of the reaction mixture for the self-assembly from PdPy*4(BF4)2 and a racemic mixture of 1S and 1R measured at 3 h are provided in Supplementary Fig. 5 and the diffusion coefficients of the observed species are summarized in Supplementary Table 16.
Mass spectrometry
A 1.92 mM solution of 1S or a 1:1 mixture of 1S and 1R-d6 (in both cases, the total amount of 1 was 0.960 μmol) in CD3NO2 (380 μL) and CD2Cl2 (120 μL), a 6.0 mM solution of 1,3,5-trimethoxybenzene in CD3NO2 (50 μL, 0.30 μmol) and a 9.6 mM solution of PdPy*4(BF4)2 in CD3NO2 (50 μL, 0.48 μmol) were mixed. At each time, 25 μL of the reaction mixture was taken, diluted with CH3NO2 (500 μL), filtered through a membrane filter (pore size: 0.20 μm) and injected into the mass spectrometer with 4.0 μL/min flow rate to obtain ESI-TOF mass spectra (Supplementary Figs 8–11). A list of predominantly observed species is shown in Supplementary Tables 17 and 18.
Quantitative analysis of the mass signal of [Pd214]4+
The ratio of [Pd21S4]4+, [Pd21S3(1R-d6)]4+, [Pd21S2(1R-d6)2]4+, [Pd21S(1R-d6)3]4+, and [Pd2(1R-d6)4]4+ is indicated by c:ca1:ca2:ca3:c. Assuming that the ionization efficiency of each species is the same and a1 and a3 are the same, the theoretical signal pattern of a mixture of [Pd214]4+ in a c:ca1:ca2:ca1:c ratio was calculated as the sum of the theoretical mass pattern of each species. Then c, a1 and a2 were determined so as to minimize the sum of the squares of the differences in the intensities of the signals between the theoretical and the experimental mass patterns and the parameter X was calculated using Eq. (8). The results are shown in Supplementary Fig. 12 and Supplementary Table 19.
Molecular modelling
Geometry optimizations of the Pd214 cages ([Pd21S4]4+, [Pd21S31R]4+, [cis-Pd21S21R2]4+ and [trans-Pd21S21R2]4+) were performed by molecular mechanics (MM) calculation with Universal Force field (BIOVIA Material Studio 2017 R2, Accelrys Software Inc.). The energy-minimized structures are provided in Supplementary Fig. 13 and their energies are summarized in Supplementary Table 20.
Data availability
The authors declare that all the other data supporting the findings of this study are available within the article and its supplementary information files and from the corresponding author upon request.
References
Barton, J. K. Metals and DNA: molecular left-handed complements. Science 233, 727–734 (1986).
Hoehlig, K., Bethge, L. & Klussmann, S. Stereospecificity of oligonucleotide interactions revisited: no evidence for heterochiral hybridization and ribozyme/DNAzyme activity. PLoS ONE 10, e0115328 (2015).
Mandal, P. K., Collie, G. W., Kauffmann, B. & Huc, I. Racemic DNA crystallography. Angew. Chem. Int. Ed. 53, 14424–14427 (2014).
Smith, C. K. & Regan, L. Construction and design of β-sheets. Acc. Chem. Res. 30, 153–161 (1997).
Gellman, S. H. Minimal model systems for β-sheet secondary structure in proteins. Curr. Opin. Chem. Biol. 2, 717–725 (1998).
Rubinstein, I., Eliash, R., Bolbach, G., Weissbuch, I. & Lahav, M. Racemic β sheets in biochirogenesis. Angew. Chem. Int. Ed. 46, 3710–3713 (2007).
Durani, S. Protein design with L- and D-α-amino acid structures as the alphabet. Acc. Chem. Res. 41, 1301–1308 (2008).
Chakrabarti, P. & Pal, D. The interrelationships of side-chain and main-chain conformations in proteins. Prog. Biophys. Mol. Biol. 76, 1–102 (2001).
Liu, M., Zhang, L. & Wang, T. Supramolecular chirality in self-assembled systems. Chem. Rev. 115, 7304–7397 (2015).
Wang, W., Wang, Y.-X. & Yang, H.-B. Supramolecular transformations within discrete coordination-driven supramolecular architectures. Chem. Soc. Rev. 45, 2656–2693 (2016).
Jędrzejewska, H. & Szumna, A. Making a right or left choice: chiral self-sorting as a tool for the formation of discrete complex structures. Chem. Rev. 117, 4863–4899 (2017).
Chen, L.-J., Yang, H.-B. & Shionoya, M. Chiral metallosupramolecular architectures. Chem. Soc. Rev. 46, 2555–2576 (2017).
Smith, D. K. Lost in translation? Chirality effects in the self-assembly of nanostructured gel-phase materials. Chem. Soc. Rev. 38, 684–694 (2009).
Szumna, A. Inherently chiral concave molecules – from synthesis to applications. Chem. Soc. Rev. 39, 4274–4285 (2010).
Gingras, M., Félix, G. & Peresutti, R. One hundred years of helicene chemistry. Part 2: stereoselective syntheses and chiral separations of carbohelicenes. Chem. Soc. Rev. 42, 1007–1050 (2013).
Meyer-Eppler, G., Topić, F., Schnakenburg, G., Rissanen, K. & Lützen, A. Chiral self-sorting of trans-chelating chiral ligands upon formation of PdII complexes. Eur. J. Inorg. Chem. 2495–2501 (2014).
Arnold, P. L., Buffet, J.-C., Blaudeck, R., Sujecki, S. & Wilson, C. Ligand recognition processes in the formation of homochiral C 3-symmetric LnL3 complexes of a chiral alkoxide. Chem. Eur. J. 15, 8241–8250 (2009).
Horie, M., Ousaka, N., Taura, D. & Yashima, E. Chiral tether-mediated stabilization and helix-sense control of complementary metallo-double helices. Chem. Sci. 6, 714–723 (2015).
Gütz, C. et al. Self-sorting effects in the self-assembly of metallosupramolecular rhombi from chiral BINOL-derived bis(pyridine) ligands. Eur. J. Org. Chem. 206–216 (2014).
Hovorka, R. et al. Self-assembly of metallosupramolecular rhombi from chiral concave 9,9′-spirobi-fluorene-derived bis(pyridine) ligands. Beilstein. J. Org. Chem. 10, 432–441 (2014).
Janczak, J. et al. Trinuclear cage-like ZnII macrocyclic complexes: enantiomeric recognition and gas adsorption properties. Chem. Eur. J. 22, 598–609 (2016).
Schaly, A., Rousselin, Y., Chambron, J.-C., Aubert, E. & Espinosa, E. The stereoselective self-assembly of chiral metallo-organic cryptophanes. Eur. J. Inorg. Chem. 832–843 (2016).
Gütz, C. et al. Enantiomerically pure [M6L12] or [M12L24] polyhedra from flexible bis(pyridine) ligands. Angew. Chem. Int. Ed. 53, 1693–1698 (2014).
Pritchard, V. E. et al. Homochiral self-sorted and emissive IrIII metallo-cryptophanes. Chem. Eur. J. 23, 6290–6294 (2017).
Costero, A. M., Colera, M., Gaviña, P., Gil, S. & Ochando, L. E. Experimental evidence for the homochiral aggregation of ammonium salts in solution. New J. Chem. 30, 1263–1266 (2006).
Kuberski, B. & Szumna, A. A self-assembled chiral capsule with polar interior. Chem. Commun. 1959–1961 (2009).
Saito, N., Terakawa, R. & Yamaguchi, M. Synthesis, π-face-selective aggregation, and π-face chiral recognition of configurationally stable C 3-symmetric propeller-chiral molecules with a π-core. Chem. Eur. J. 20, 5601–5607 (2014).
Safont-Sempere, M. M. et al. Impact of molecular flexibility on binding strength and self-sorting of chiral π-surfaces. J. Am. Chem. Soc. 133, 9580–9591 (2011).
Ponnuswamy, N., Cougnon, F. B. L., Clough, J. M., Pantoş, G. D. & Sanders, J. K. M. Discovery of an organic trefoil knot. Science 338, 783–785 (2012).
Makiguchi, W. et al. Chirality- and sequence-selective successive self-sorting via specific homo- and complementary-duplex formations. Nat. Commun. 6, 7236 (2015).
Jędrzejewska, H., Wierzbicki, M., Cmoch, P., Rissanen, K. & Szumna, A. Dynamic formation of hybrid peptidic capsules by chiral self-sorting and self-assembly. Angew. Chem. Int. Ed. 53, 13760–13764 (2014).
Szymański, M. et al. Mechanochemical encapsulation of fullerenes in peptidic containers prepared by dynamic chiral self-sorting and self-assembly. Chem. Eur. J. 22, 3148–3155 (2016).
Tsiamantas, C. et al. Selective dynamic assembly of disulfide macrocyclic helical foldamers with remote communication of handedness. Angew. Chem. Int. Ed. 55, 6848–6852 (2016).
Petryk, M., Biniek, K., Janiak, A. & Kwit, M. Unexpected narcissistic self-sorting at molecular and supramolecular levels in racemic chiral calixsalens. CrystEngComm 18, 4996–5003 (2016).
Sisco, S. W. & Moore, J. S. Homochiral self-sorting of BINOL macrocycles. Chem. Sci. 5, 81–85 (2014).
Telfer, S. G. et al. Mono- and dinuclear complexes of chiral tri- and tetradentate Schiff-base ligands derived from 1,1′-binaphthyl-2,2′-diamine. Inorg. Chem. 43, 6168–6176 (2004).
Bunzen, J., Bruhn, T., Bringmann, G. & Lützen, A. Synthesis and helicate formation of a new family of BINOL-based bis(bipyridine) ligands. J. Am. Chem. Soc. 131, 3621–3630 (2009).
Gidron, O., Ebert, M.-O., Trapp, N. & Diederich, F. Chiroptical detection of nonchromophoric, achiral guests by enantiopure alleno-acetylenic helicages. Angew. Chem. Int. Ed. 53, 13614–13618 (2014).
Gütz, C. et al. Enantiomerically pure trinuclear helicates via diastereoselective self-assembly and characterization of their redox chemistry. J. Am. Chem. Soc. 136, 11830–11838 (2014).
Gidron, O. et al. Homochiral [2]catenane and bis[2]catenane from alleno-acetylenic helicates-a highly selective narcissistic self-sorting process. J. Am. Chem. Soc. 137, 12502–12505 (2015).
Yan, L.-L. et al. Stereocontrolled self-assembly and self-sorting of luminescent europium tetrahedral cages. J. Am. Chem. Soc. 137, 8550–8555 (2015).
Rizzuto, F. J. & Nitschke, J. R. Stereochemical plasticity modulates cooperative binding in a CoII 12L6 cuboctahedron. Nat. Chem. 9, 903–908 (2017).
Klein, C. et al. A new structural motif for an enantiomerically pure metallosupramolecular Pd4L8 aggregate by anion templating. Angew. Chem. Int. Ed. 53, 3739–3742 (2014).
Naito, M., Souda, H., Koori, H., Komiya, N. & Naota, T. Binuclear trans-bis(β-iminoaryloxy)palladium(II) complexes doubly linked with pentamethylene spacers: structure-dependent flapping motion and heterochiral association behavior of the clothespin-shaped molecules. Chem. Eur. J. 20, 6991–7000 (2014).
Weilandt, T., Kiehne, U., Schnakenburg, G. & Lützen, A. Diastereoselective self-assembly of dinuclear heterochiral metallosupramolecular rhombs in a self-discriminating process. Chem. Commun. 2320–2322 (2009).
Mizumura, M., Shinokubo, H. & Osuka, A. Synthesis of chiral porphyrins through Pd-catalyzed [3+2] annulation and heterochiral self-assembly. Angew. Chem. Int. Ed. 47, 5378–5381 (2008).
Claessens, C. G. & Torres, T. Chiral self-discrimination in a M3L2 subphthalocyanine cage. J. Am. Chem. Soc. 124, 14522–14523 (2002).
Henkelis, J. J. et al. Metallo-cryptophanes decorated with bis-N-heterocyclic carbene ligands: self-assembly and guest uptake into a nonporous crystalline lattice. J. Am. Chem. Soc. 136, 14393–14396 (2014).
Wu, A. & Isaacs, L. Self-sorting: the exception or the rule? J. Am. Chem. Soc. 125, 4831–4835 (2003).
Sun, Q. F., Sato, S. & Fujita, M. An M12(L1)12(L2)12 cantellated tetrahedron: a case study on mixed-ligand self-assembly. Angew. Chem. Int. Ed. 53, 13510–13513 (2014).
Ronson, T. K., Roberts, D. A., Black, S. P. & Nitschke, J. R. Stacking interactions drive selective self-assembly and self-sorting of pyrene-based MII 4L6 architectures. J. Am. Chem. Soc. 137, 14502–14512 (2015).
Wu, A., Chakraborty, A., Fettinger, J. C., Flowers, R. A. & Isaacs, L. Molecular clips that undergo heterochiral aggregation and self-sorting. Angew. Chem. Int. Ed. 41, 4028–4031 (2002).
Maeda, T. et al. Double-stranded helical polymers consisting of complementary homopolymers. J. Am. Chem. Soc. 130, 7938–7945 (2008).
Hiraoka, S. What do we learn from the molecular self-assembly process? Chem. Rec. 15, 1144–1147 (2015).
Kai, S. et al. Quantitative analysis of self-assembly process of a Pd2L4 cage consisting of rigid ditopic ligands. Chem. Eur. J. 24, 663–671 (2018).
Kai, S. et al. Flexibility of components alters the self-assembly pathway of Pd2L4 coordination cages. Dalton Trans. 47, 3258–3283 (2018).
Tsujimoto, Y., Kojima, T. & Hiraoka, S. Rate-determining step in the self-assembly process of supramolecular coordination capsules. Chem. Sci. 5, 4167–4172 (2014).
Kai, S. et al. The effect of solvent and coordination environment of metal source on the self-assembly pathway of a Pd(II)-mediated coordination capsule. Inorg. Chem. 56, 12652–12663 (2017).
Kai, S. et al. Steric interaction between neighboring components favors the formation of large intermediates in the self-assembly process of a Pd2L4 capsule. Chem. Eur. J. 24, 3965–3669 (2018).
Kai, S., Shigeta, T., Kojima, T. & Hiraoka, S. Quantitative analysis of self-assembly process of a Pd12L24 coordination sphere. Chem. Asian J. 12, 3203–3207 (2017).
Tateishi, T. et al. Self-assembly of a Pd4L8 double-walled square partly takes place through the formation of kinetically trapped species. Eur. J. Inorg. Chem. 1192–1197 (2018).
Baba, A., Kojima, T. & Hiraoka, S. Self-assembly process of dodecanuclear Pt(II)-linked cyclic hexagon. J. Am. Chem. Soc. 137, 7664–7667 (2015).
Baba, A., Kojima, T. & Hiraoka, S. Quantitative analysis of self-assembly process of hexagonal Pt(II) macrocyclic complexes: effect of solvent and components. Chem. Eur. J. 24, 838–847 (2018).
Gütz, C., Hovorka, R., Schnakenburg, G. & Lützen, A. Homochiral supramolecular M2L4 cages by high-fidelity self-sorting of chiral ligands. Chem. Eur. J. 19, 10890–10894 (2013).
Tobe, M. L. & Burgess, J. Inorganic Reaction Mechanisms (Longman: London, U.K., 1999).
Jiang, W., Schäfer, A., Mohr, P. C. & Schalley, C. A. Monitoring self-sorting by electrospray ionization mass spectrometry: formation intermediates and error-correction during the self-assembly of multiply threaded pseudorotaxanes. J. Am. Chem. Soc. 132, 2309–2320 (2010).
Zheng, Y.-R. & Stang, P. J. Direct and quantitative characterization of dynamic ligand exchange between coordination-driven self-assembled supramolecular polygons. J. Am. Chem. Soc. 131, 3487–3489 (2009).
Lv, Y. Method for preparing dimethoxymethane. China patent CN105646165A (2016).
Berliner, M. & Belecki, K. Synthesis of alpha-halo ethers from symmetric acetals and in situ methoxymethylation of an alcohol. Org. Synth. 84, 102–110 (2007).
Acknowledgements
This research was supported by JSPS Grants-in-Aid for Scientific Research on Innovative Areas “Dynamical Ordering of Biomolecular Systems for Creation of Integrated Functions” (25102001 and 25102005) and The Asahi Glass Foundation.
Author information
Authors and Affiliations
Contributions
T.T., T.K. and S.H. conceived the project. S.H. prepared the manuscript and all the authors discussed the results and commented on the manuscript. T.T. synthesized all the ditopic ligands (1S, racemic mixture of 1S and 1R and 1R-d6) carried out all the NMR and ESI-TOF mass measurements. T.T., T.K. and S.H. analysed the experimental data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tateishi, T., Kojima, T. & Hiraoka, S. Chiral self-sorting process in the self-assembly of homochiral coordination cages from axially chiral ligands. Commun Chem 1, 20 (2018). https://doi.org/10.1038/s42004-018-0020-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-018-0020-4
This article is cited by
-
Stereo-control on Lanthanide Triple-stranded Helicates Toward Enhanced Enantioselective Sensing
Chemical Research in Chinese Universities (2024)
-
Nonconventional driving force for selective oxidative C–C coupling reaction due to concurrent and curious formation of Ag0
Scientific Reports (2021)
-
Self-assembly process of a quadruply interlocked palladium cage
Communications Chemistry (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.