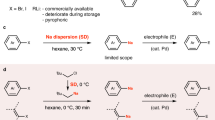
Abstract
Sodium is the most abundant alkali metal in the Earth’s crust and the ocean. However, organosodium compounds have long been considered inferior to organolithium compounds, which have instead dominated synthetic organic chemistry during the last century. Despite being largely neglected because of their reactive nature, it is worth re-exploring organosodium chemistry, in light of the growing demand for sustainable syntheses without recourse to less abundant elements such as lithium. Herein, we demonstrate that, contrary to common belief, organosodium compounds can be easily prepared from aryl chlorides or (hetero)arenes and easy-to-handle sodium dispersion and, after being transmetallated to the corresponding zinc and boron compounds, they readily participate in the Negishi and Suzuki–Miyaura cross-coupling reactions, fundamental carbon–carbon bond-forming reactions in organic synthesis. Direct coupling reactions with organosodium species were also possible.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Seyferth, D. Alkyl and aryl derivatives of the alkali metals: useful synthetic reagents as strong bases and potent nucleophiles. 1. Conversion of organic halides to organoalkali-metal compounds. Organometallics 25, 2–24 (2006).
Seyferth, D. Alkyl and aryl derivatives of the alkali metals: strong bases and reactive nucleophiles. 2. Wilhelm Schlenkas organoalkali-metal chemistry. The metal displacement and the transmetalation reactions. Metalation of weakly acidic hydrocarbons. Superbases. Organometallics 28, 2–33 (2009).
Schlosser, M. (ed.) Organometallics in Synthesis: A Manual 2nd edn (Wiley, Chichester, 2002).
Nobis, J. F., Moormeier, L. F. & Robinson, R. E. Organosodium compounds for preparation of other carbon–metal bonds. Adv. Chem. Ser. 23, 63–68 (1959).
Bockmühl, M. & Ehrhart, G. Sodium phenyl and its derivatives and process of preparing them. Reichspatent 622,875 (1935) and US patent 2,012,372 (1935).
Gilman, H. & Wright, G. F. The mechanism of the Wurtz–Fittig reaction. The direct preparation of an organosodium (potassium) compound from an RX compound. J. Am. Chem. Soc. 55, 2893–2896 (1933).
Benkeser, R. A., Foster, D. J., Sauve, D. M. & Nobis, J. F. Metalations with organosodium compounds. Chem. Rev. 57, 867–894 (1957).
Schlosser, M. Organosodium and organopotassium compounds. Part I: Properties and reactions. Angew. Chem. Int. Ed. 3, 287–306 (1964).
Schlosser, M. Organosodium and organopotassium compounds. Part II: Preparation and synthetic applications. Angew. Chem. Int. Ed. 3, 362–373 (1964).
Gissot, A. et al. Directed ortho-metalation, a new insight into organosodium chemistry. Angew. Chem. Int. Ed. 41, 340–343 (2002).
Ma, Y., Algera, R. F. & Collum, D. B. Sodium diisopropylamide in N,N-dimethylethylamine: reactivity, selectivity, and synthetic utility. J. Org. Chem. 81, 11312–11315 (2016).
Huang, Y., Chan, G. H. & Chiba, S. Amide-directed C–H sodiation by a sodium hydride/iodide composite. Angew. Chem. Int. Ed. 56, 6544–6547 (2017).
Weidmann, N., Ketels, M. & Knochel, P. Sodiation of arenes and heteroarenes in continuous flow. Angew. Chem. Int. Ed. 57, 10748–10751 (2018).
The Nobel Prize in Chemistry 2010. The Nobel Prize. https://www.nobelprize.org/prizes/chemistry/2010/summary/ (2010).
Johansson Seechurn, C. C. C., Kitching, M. O., Colacot, T. J. & Snieckus, V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 51, 5062–5085 (2012).
de Meijere, A., Bräse, S. & Oestreich, M. (eds) Metal-Catalyzed Cross-Coupling Reactions and More Vols 1, 2 and 3 (Wiley-VCH, Weinheim, 2014).
Negishi, E. Magical power of transition metals: past, present, and future. Angew. Chem. Int. Ed. 50, 6738–6764 (2011).
Krasovskiy, A. & Knochel, P. A LiCl-mediated Br/Mg exchange reaction for the preparation of functionalized aryl- and heteroarylmagnesium compounds from organic bromides. Angew. Chem. Int. Ed. 43, 3333–3336 (2004).
Nakamura, E. & Sato, K. Managing the scarcity of chemical elements. Nat. Mater. 10, 158–161 (2011).
Yabuuchi, N., Kubota, K., Dahbi, M. & Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 114, 11636–11682 (2014).
Vesborg, P. C. K. & Jaramillo, T. F. Addressing the terawatt challenge: scalability in the supply of chemical elements for renewable energy. RSC Adv. 2, 7933–7947 (2012).
Nobis, J. F. & Moormeier, L. F. Phenylsodium route to phenylacetic acid and dimethyl phenylmalonate. Ind. Eng. Chem. 46, 539–544 (1954).
Screttas, C. G., Steele, B. R., Micha-Screttas, M. & Heropoulos, G. A. Aryllithiums with increasing steric crowding and lipophilicity prepared from chlorides in diethyl ether. The first directly prepared room-temperature-stable dilithioarenes. Org. Lett. 14, 5680–5683 (2012).
Valente, C. et al. The development of bulky palladium NHC complexes for the most-challenging cross-coupling reactions. Angew. Chem. Int. Ed. 51, 3314–3332 (2012).
Kawamorita, S., Ohmiya, H., Iwai, T. & Sawamura, M. Palladium-catalyzed borylation of sterically demanding aryl halides with a silica-supported compact phosphane ligand. Angew. Chem. Int. Ed. 50, 8363–8366 (2011).
Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C–H activation for the construction of C–B bonds. Chem. Rev. 110, 890–931 (2010).
Giannerini, M., Fañanás-Mastral, M. & Feringa, B. L. Direct catalytic cross-coupling of organolithium compounds. Nat. Chem. 5, 667–672 (2013).
Pinxterhuis, E. B., Giannerini, M., Hornillos, V. & Feringa, B. L. Fast, greener and scalable direct coupling of organolithium compounds with no additional solvents. Nat. Commun. 7, 11698 (2016).
Jia, Z., Liu, Q., Peng, X.-S. & Wong, H. N. C. Iron-catalysed cross-coupling of organolithium compounds with organic halides. Nat. Commun. 7, 10614 (2016).
Leiendecker, M., Hsiao, C.-C., Guo, L., Alandini, N. & Rueping, M. Metal-catalyzed dealkoxylative Caryl–Csp3 cross-coupling—replacement of aromatic methoxy groups of aryl ethers by employing a functionalized nucleophile. Angew. Chem. Int. Ed. 53, 12912–12915 (2014).
Yang, Z.-K. et al. Cross-coupling of organolithium with ethers or aryl ammonium salts by C–O or C–N bond cleavage. Chem. Eur. J. 22, 15693–15699 (2016).
Giannerini, M., Hornillos, V., Vila, C., Fañanás-Mastral, M. & Feringa, B. L. Hindered aryllithium reagents as partners in palladium-catalyzed cross-coupling: synthesis of tri- and tetra-ortho-substituted biaryls under ambient conditions. Angew. Chem. Int. Ed. 52, 13329–13333 (2013).
Hornillos, V., Giannerini, M., Vila, C., Fañanás-Mastral, M. & Feringa, B. L. Catalytic direct cross-coupling of organolithium compounds with aryl chlorides. Org. Lett. 15, 5114–5117 (2013).
Ehrhart, G. Über umsetzungen mit phenylnatrium. Chem. Ber. 96, 2042–2046 (1963).
Becht, J.-M., Gissot, A., Wagner, A. & Mioskowski, C. An efficient synthesis of biaryls via noncatalysed anionic coupling of an arylsodium with haloarenes. Tetrahedron Lett. 45, 9331–9333 (2004).
Acknowledgements
The authors thank Okayama University and KOBELCO ECO-Solutions Co., Ltd for financial support.
Author information
Authors and Affiliations
Contributions
S.A. and K.T. conceived and designed the experiments. H.N. performed the experiments. S.A. and K.T. prepared the manuscript. All authors contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
S.A. and K.T. are listed as inventors on patent applications (JP2017/247538, JP2018/005719, JP2018/099899) that cover the cross-coupling reactions presented in this paper.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–4, Supplementary References
Rights and permissions
About this article
Cite this article
Asako, S., Nakajima, H. & Takai, K. Organosodium compounds for catalytic cross-coupling. Nat Catal 2, 297–303 (2019). https://doi.org/10.1038/s41929-019-0250-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0250-6
This article is cited by
-
Synthesis of trans-1,2-dimetalloalkenes through reductive anti-dimagnesiation and dialumination of alkynes
Nature Synthesis (2023)
-
Continuous Flow Generation of Highly Reactive Organometallic Intermediates: A Recent Update
Journal of Flow Chemistry (2023)
-
Exchange made easy
Nature Reviews Chemistry (2021)
-
Halogen–sodium exchange enables efficient access to organosodium compounds
Communications Chemistry (2021)
-
Conversion of triphenylphosphine oxide to organophosphorus via selective cleavage of C-P, O-P, and C-H bonds with sodium
Communications Chemistry (2020)