Abstract
Given the pleiotropic effects of statins beyond their lipid-lowering effects, there have been attempts to evaluate the role of statin therapy in IPF, but they have shown inconclusive results. Data from the National Health Insurance Service (NHIS) database of South Korea were used to investigate the effects of statin therapy on IPF. The IPF cohort consisted of a total of 10,568 patients who were newly diagnosed with IPF between 2010 and 2017. These patients were then matched in a 1:3 ratio to 31,704 subjects from a control cohort without IPF, with matching based on age and sex. A case–control study was performed to evaluate the association between statin use and the risk for IPF, and the multivariable analysis revealed that statin use was associated with a lower risk for IPF (adjusted OR 0.847, 95% CI 0.800–0.898). Using the IPF cohort, we also evaluated whether statin use at the time of diagnosis was associated with future clinical outcomes. The statin use at the time of IPF diagnosis was associated with improved overall survival (adjusted HR 0.779, 95% CI 0.709–0.856). Further prospective studies are needed to clarify the role of statin therapy in IPF.
Similar content being viewed by others
Introduction
Idiopathic pulmonary fibrosis (IPF) is a prototype of progressive fibrotic lung disease. Progressive fibrosis is mainly driven by repetitive microinjuries to the alveolar epithelium, leading to aberrant repair process1. Although the development of anti-fibrotic drugs has slowed the decline in lung function, there is still an unmet need to improve the prognosis of patients with IPF2.
While aberrant epithelial-mesenchymal crosstalk is regarded to be the key pathogenetic factor in IPF, inflammation is also considered to play a role3. Currently approved anti-fibrotic drugs for IPF, such as pirfenidone and nintedanib, have anti-inflammatory effects in addition to their well-known anti-fibrotic effects4,5. Although aggressive immunosuppressive therapy has been shown to be potentially harmful in IPF6, an adequate level of control of inflammatory responses may be helpful3.
Statins are widely used to treat dyslipidemia by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. In addition to lipid-lowering effects, statins have anti-inflammatory and anti-oxidative effects7. In this regard, the role of statins in IPF has been evaluated in a few studies. Some studies have reported beneficial effects of statins in delaying disease progression and reducing mortality8,9,10,11, while others have not12. A recent meta-analysis also failed to provide conclusive evidence supporting the beneficial effects of statins in IPF13. Furthermore, most available studies have focused on the effects of statins in patients already diagnosed with IPF. Data regarding whether the use of statins could alter the risk for IPF in the general population or subjects at risk for IPF are lacking, including those with interstitial lung abnormality (ILA).
As such, we aimed to investigate the role of statin therapy in IPF and designed two distinct types of studies for this purpose. First, we conducted a case–control study to evaluate the association between statin use and the risk for IPF by comparing patients with IPF and control subjects without IPF. Second, we conducted a retrospective cohort study focusing only on the cohort of patients with IPF to evaluate whether clinical outcomes differed between statin users and nonusers among patients with IPF. A nationwide, population-based cohort data from the National Health Insurance Service (NHIS) database of South Korea was used for this study.
Results
Characteristics of the study population
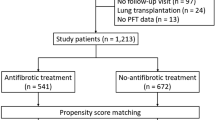
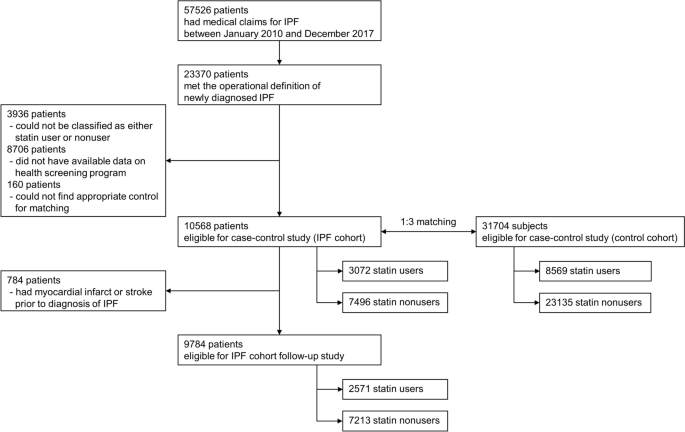
A search of the NHIS database identified 23,370 patients newly diagnosed with IPF during the study period. After excluding patients in whom the operational definition of statin use could not be applied (i.e., only transient use) and those who did not undergo health screening programs within 2 years before the IPF diagnosis, 10,568 patients were ultimately included in the IPF cohort. For this IPF cohort, a control cohort comprising 31,704 subjects without IPF were matched at a ratio of 1:3 according to age and sex (Fig. 1). The mean age of the patients was 65 years, and 69% were male. Comorbidities related to cardiovascular diseases were more common in the IPF cohort, including diabetes and ischemic heart disease (Table 1).
Statin use and the risk for IPF
Among the 10,568 patients in the IPF cohort, 3072 patients (29.1%) were statin users and, among the 31,704 subjects in the control cohort, 8569 subjects (27.0%) were statin users. When adjusted for covariates including comorbidities, statin use was associated with a lower risk for IPF (adjusted OR 0.847, 95% CI 0.800–0.898) than statin nonuse. The protective effects of statin use were consistent regardless of smoking history and sex (Table 2).
Statin use and clinical outcomes of IPF
Among the 10,568 patients in the IPF cohort, 9784 patients who did not experience myocardial infarction or stroke before the diagnosis of IPF were analyzed to assess the effects of statins on clinical outcomes (Fig. 1). Significant differences were observed in baseline characteristics between statin users and nonusers (Table 3). Statin users were older and cardiovascular comorbidities, such as hypertension and diabetes, were significantly more common among statin users, with almost more than twice the frequency.
The clinical outcomes of the two groups were compared and analyzed (Table 4). When adjusted for covariates, statin users exhibited lower overall mortality than statin nonusers (adjusted HR 0.779, 95% CI 0.709–0.856). When the time to first hospitalization or ER visit for any cause was analyzed, no significant difference was observed between the two groups (adjusted HR 0.974, 95% CI 0.908–1.044). However, the risk for hospitalization or ER visits due to respiratory causes was lower in statin users than in statin nonusers (adjusted HR 0.818, 95% CI 0.728–0.920).
Impact of duration of statin use
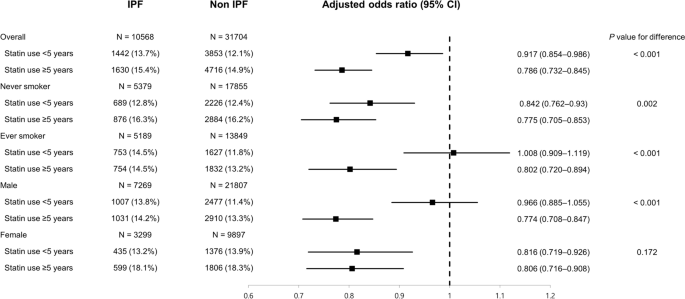
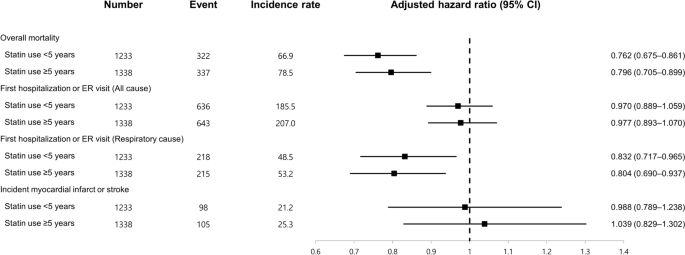
We evaluated whether the duration of statin use influenced the association between statin use and the risk for and clinical outcomes of IPF (Fig. 2). When statin nonusers were regarded as the reference group, statin use ≥ 5 years was associated with a lower risk for IPF (adjusted OR 0.786, 95% CI 0.732–0.845) than statin use < 5 years (adjusted OR 0.917, 95% CI 0.854–0.986), and the difference was statistically significant (P < 0.001). This trend was more pronounced in ever-smokers (P < 0.001) and male subjects (P < 0.001). In particular, in the subgroup of ever-smokers and male subjects, statin use < 5 years was not associated with a lower risk for IPF than statin nonusers (adjusted OR 1.008, 95% CI 0.909–1.119 and 0.966, 95% CI 0.885–1.055, respectively). However, when an analysis was performed to evaluate the association between statin use and clinical outcomes, the degrees of association were similar regardless of the duration of statin use (Fig. 3).
Discussion
In this study, we evaluated the association between statin use and the risk for IPF, and the effects of statin use on the clinical outcomes of IPF, using large-scale, nationwide cohort data. When case–control analysis was performed by matching patients with IPF with the control cohort according to age and sex, statin use was associated with a lower risk for IPF than statin nonuse. In addition, in the IPF cohort, statin use was associated with a lower risk for overall mortality and first hospitalization or ER visits related to respiratory causes.
When investigating the causes or risk factors for a specific disease, a longitudinal prospective cohort study involving healthy subjects and monitoring the development of the disease is the best option. However, for rare diseases with low incidence, such as IPF, it is not easy to perform such a study because it would require a very large number of subjects and a follow-up period of several decades, hence a case–control study is a good alternative14. As such, we performed a case–control study to investigate whether statin use could alter the risk for IPF and demonstrated promising results, suggesting a protective role of statins against IPF.
Repetitive alveolar microinjuries play a key role in the pathogenesis of IPF and result from multiple genetic and environmental risk factors15. While diabetes has been suggested to be associated with IPF16, metformin, a representative drug for diabetes, was shown to attenuate pulmonary fibrosis in an in-vivo model17. However, data regarding whether a specific drug can modify the risk for IPF are scarce. In our case–control analysis, statin use was associated with a lower risk for IPF than statin nonuse. Considering that statins have a favorable safety profile and are widely used in the general population, further studies should be performed to determine whether our findings are reproducible.
Nevertheless, our study has a limitation in that we used a dataset consisting of case and control cohorts matched only for age and sex. There may have been unmeasured biases despite our effort to adjust for confounding factors through multivariable analysis. Although incorporating all available variables for matching would have resulted in more balanced samples, this was practically unattainable because we could not have access to the complete NHIS dataset covering the entire Korean population.
In addition, our study is limited in that we were unable to determine the precise timeframe required for statins to exert a certain pharmacologic effect on the risk of developing IPF. While we attempted to provide a clear definition of statin exposure by classifying patients based on whether statins were prescribed within 1 year before their diagnosis of IPF, we recognize the limitation that statin use for a wide range of durations would have been grouped together as statin users. Exposure to statins over only a few years may not be sufficient to significantly influence the risk of IPF. To address this concern, we performed a subgroup analysis based on the duration of statin use, which revealed that statin use ≥ 5 years conferred a greater protective effect regarding the risk of IPF compared with statin use < 5 years. It is likely that a longer period of time is required for a particular drug to modify the risk of IPF, as supported by a recent study showing that approximately 3 years was required for significant progression of ILA18.
Recently, ILA has been increasingly detected and recognized on chest computed tomography among asymptomatic population. ILA is classified into fibrotic and non-fibrotic types, and fibrotic ILA can be a potential precursor to IPF19. However, it remains unclear how to prevent disease progression in the subclinical status of ILA, and systematic close follow-up is recommended for high-risk subjects20. Currently approved anti-fibrotic drugs, pirfenidone and nintedanib, would not be appropriate in the setting of ILA, given the non-negligible costs and potential drug toxicities. The results of our study suggest that statins may merit investigation of their role in preventing the progression of ILA to IPF.
The protective effects of statins against IPF are biologically plausible in several respects. IPF is known to be a disease of the aging lung, with telomere shortening as one of the contributing factors, which makes the lungs more susceptible to maladaptive responses to alveolar micro-injuries21. There is some evidence that statins have anti-aging effects linked to their ability to inhibit telomere shortening22. These pleiotropic effects of statins, beyond their lipid-lowering effects, may play a protective role against the development of IPF. Interestingly, in our study, statin use ≥ 5 years was associated with a lower risk for IPF in ever-smokers, whereas statin use < 5 years was not. Given the results of previous studies reporting that cigarette smoking could result in shorter telomeres23, longer use of statins may be needed to offset the detrimental effects of cigarette smoking in ever-smokers.
Among patients with newly diagnosed IPF, statin use at baseline was associated with improvement in clinical outcomes in our study compared to statin nonuse, especially for overall survival. There have been a few attempts to evaluate the impact of statin therapy on clinical outcomes in IPF, but a meta-analysis of these studies could not draw a definitive conclusion on the effects of statin use on overall mortality13. In fact, most of these previous studies had limited sample sizes, usually including hundreds of patients8,9,10,11,12. Our study included approximately 10,000 patients with IPF, which provided greater statistical power. Although statin users had more cardiovascular comorbidities, which was not surprising, when these confounding factors were adjusted for, statin use was associated with lower overall mortality. However, there is a possibility of the presence of unmeasured confounding factors in our analysis. Because our analysis was retrospective and relied on the NHIS database, which is a health claims database, this study cannot completely eliminate every potential bias. To fully address this concern, it would be imperative to perform a propensity score-matched analysis within a meticulously established cohort of patients with IPF or, ideally, to conduct a well-designed prospective study.
There is some experimental evidence supporting the anti-fibrotic potential of statins24,25,26, which are also known to have anti-inflammatory effects27. Both anti-fibrotic and anti-inflammatory effects may be beneficial in slowing the progression of IPF. However, in our study, although we demonstrated that overall survival was better in statin users among patients with IPF, it is not clear whether this finding was due to differences in IPF-related mortality or mortality related to other causes, because we could not determine the cause of death in this study. Considering that cardiovascular diseases and lung cancers are also the main causes of death in patients with IPF28, the beneficial effects of statins on overall mortality may stem from their impact on these conditions. Statins are well-known to have cardiovascular protective effects and, in addition, a recent study using the Taiwan national health insurance database reported that statin use was associated with lower risk for lung cancers in patients with interstitial lung diseases29. However, our finding that statin use was associated with a lower risk for events caused by respiratory causes indicates that statins may have beneficial effects on IPF itself.
The strength of our study is that we used representative nationwide data from a large number of patients captured from real-world clinical practice. However, this study had some limitations that should be addressed. First, although our study suggested a protective role of statins against the risk for IPF, a case–control study could not confirm a causal relationship. The effects of statins on the prognosis of patients with IPF could not be definitively addressed in our study due to its retrospective design. As such, a well-designed, prospective study should be conducted in the future. Second, about 50% of patients who met the operational diagnosis of newly diagnosed IPF was excluded in the final analysis. The most common cause of exclusion was because they did not undergo health screening program within 2 years. It can be suggested that patients with relatively higher health motivation were included in this study. Third, the NHIS database did not have information about the severity of IPF, such as lung function parameters. Therefore, we could not fully adjust for confounding factors when analyzing the clinical outcomes of patients with IPF. Fourth, we could not assess the effects of anti-fibrotic therapy. This is because in Korea, pirfenidone only began to be covered by the National Health Insurance in October 2015, and nintedanib has not yet been covered. Given that our study period was up to December 2017, we did not have sufficient data regarding the use of anti-fibrotic drugs. However, we suspect that the prescription of anti-fibrotic drugs would not have been so different, regardless of whether patients were statin users. Considering the mortality-reducing effects of anti-fibrotic therapy30, future studies should gather information about the use of anti-fibrotic drugs when evaluating whether a specific drug could improve the prognosis of IPF. Fifth, our study only investigated the impact of statin use preceding the diagnosis of IPF. Given the possibility that some patients may have started statin therapy after the diagnosis of IPF, statistical methods such as time-varying Cox regression or landmark time analysis may be useful to addressing this issue31. Sixth, the NHIS database used for this study predominantly covered only Asian populations, limiting our ability to evaluate patients of diverse ethnic backgrounds. Further studies should be performed in other cohorts from different countries. Seventh, we employed unconditional logistic regression in the analysis of our case–control matched dataset, under the premise that our matching, which was limited to only age and sex, were comparatively loose32,33. The absence of conditional matched regression in our statistical methodology raises concerns regarding potential biases in our results. Finally, because this study was based on information gathered from a database, misclassification related to statin use and/or diagnosis of IPF could have occurred. A prescription history of statins may not necessarily mean that the patient actually took the drug. However, in clinical practice, statins are usually taken continuously, unless there are serious side effects, which are relatively rare. Thus, the chances of exposure misclassification appear to be low. In addition, the diagnosis of IPF is strictly reviewed for registration in Korea, because patients with ICD-10 codes for IPF are financially supported by the government. Furthermore, we included only patients diagnosed at referral hospitals, which makes us more confident in the reliability of IPF diagnosis. However, relying solely on the health claims database and utilizing ICD codes for identifying cases with IPF presents inherent limitations, primarily due to our limited ability to verify the diagnostic accuracy.
In conclusion, this large, nationwide, population-based cohort study conducted in a real-world setting found that statin use was associated with a lower risk for IPF than statin nonuse. In addition, statin use was associated with improved overall survival and reduced risk for respiratory-related hospitalization or ER visits among patients with IPF. Prospective studies aiming to confirm the potential beneficial effects of statins on IPF are warranted.
Methods
Study data source
This study used data from the health claims database established by the NHIS of South Korea, a single insurer managed by the Korean government. Because it is mandatory for every Korean citizen to subscribe to the NHIS, the NHIS database contains extensive health-related data from the entire Korean population, including personal and sociodemographic information, data regarding every inpatient and outpatient service, prescriptions, and mortality data34.
In South Korea, the NHIS has been providing national health screening checkup programs since 1995 to improve the health status of the general population. Health-related data obtained through this health screening program are also available in the NHIS database35. The data and materials of the NHIS are accessible to the public and are widely used by medical researchers. Requirement for informed consent was waived by the institutional review board of Seoul National University Hospital because information regarding personal identification was completely removed while establishing the database. The study protocol was approved by the institutional review board of Seoul National University Hospital (IRB No. E-1904-001-1020), and all methods were carried out in accordance with relevant guidelines and regulations.
Study design and population
The present study was a retrospective analysis based on data from the NHIS database, which included two distinct parts. The first is the case–control study comparing patients with IPF and control populations, and the second is the retrospective cohort study focusing only on the IPF cohort to evaluate the beneficial effect of statins in IPF.
A case–control study was performed to evaluate the association between statin use and risk for IPF. For this purpose, a cohort of patients with newly diagnosed IPF was established. First, patients who had medical claims with International Classification of Diseases (ICD-10) codes for IPF (J841 or J8418) between January 2010 and December 2017 were screened. We chose to exclude patients diagnosed prior to 2010 given the considerable differences in treatment strategies and clinical outcomes between patients diagnosed in the earlier period and those diagnosed more recently36. Subsequently, an operational definition of newly diagnosed IPF, as detailed in our previous study, was applied37.
Briefly, we first searched for patients in whom ICD-10 codes for IPF were registered by physicians working in referral hospitals, not primary care clinics, considering that an accurate diagnosis of IPF requires multidisciplinary discussion. Among these, patients with claims for chest computed tomography (CT) and pulmonary function tests within 1 year and 6 months, respectively, before the first registration of ICD-10 codes for IPF were included. Patients with ICD-10 codes for autoimmune or connective tissue diseases, or other pulmonary diseases were excluded. Among the selected patients fulfilling this operational definition, only those who had participated in national health screening programs within 2 years before the diagnosis of IPF were considered to be eligible for this study to use the health-related information that could be obtained through the programs, such as smoking history.
Study patients were classified into statin users and nonusers according to whether they were being prescribed statins at the time of IPF diagnosis. An operational definition of statin use was applied for this purpose because the exact timeframe for statins to exert potential effects on the risk of IPF is not certain. A patient was regarded to be a statin user if statins were prescribed at least twice within 1 year before the index date, the date of the first registration of the ICD-10 codes for IPF. To define only those who had never been exposed to statins as statin nonusers, patients in whom statins were prescribed only once within 1 year before the index date or statins were ever prescribed before but not within 1 year before the index date were excluded.
Following the establishment of the IPF cohort, another control cohort without IPF, 1:3 matched according to age and sex, was established using the exact matching algorithm. The control population was selected without replication among subjects without ICD-10 codes for IPF. Statin users and nonusers were defined as those in the IPF cohort. The index date for the control population was determined as the index date for the matched case patients.
After conducting a case–control study, a subsequent analysis was performed using follow-up data from study patients in the IPF cohort as a retrospective cohort study. We evaluated the association between statin use and clinical outcomes. For this analysis, patients who had already been diagnosed with myocardial infarction or stroke before the index date were excluded because incident myocardial infarction or stroke was one of the outcome events of interest.
Study outcome and covariates
In the case–control study, the association between statin use and the risk for IPF was assessed by calculating odds ratios (ORs). After matching for age and sex, additional adjustments were performed for the following covariates: smoking history, alcohol consumption pattern, body mass index, income level, and comorbidities including diabetes, hypertension, ischemic heart disease, stroke, and chronic kidney disease. The presence of comorbidities was assessed using the relevant ICD-10 codes for each disease. Covariates were selected among clinically relevant variables that could be associated with statin use and identified through the NHIS database.
Using only the IPF cohort, we investigated whether statin use was associated with improvement in clinical outcomes, including overall mortality, hospitalization or emergency room (ER) visits, and incident myocardial infarction or stroke. After the overall events of hospitalization or ER visits were assessed, events associated with a primary diagnosis of respiratory diseases were further analyzed using ICD-10 codes of J00–J99. However, the cause of mortality could not be identified through our database. The covariates adjusted for were similar to those used in the case–control analysis and included smoking history, alcohol consumption pattern, body mass index, income level, and comorbidities including diabetes, hypertension, and chronic kidney disease.
Statistical analysis
Descriptive statistics were used to summarize the baseline characteristics of the study population. Continuous variables were summarized as means with standard deviations and categorical variables were reported as frequencies (percentages). The association between statin use and the risk for IPF was evaluated using multivariable logistic regression, and adjusted ORs were reported with corresponding 95% confidence intervals (CIs). Subgroup analysis according to smoking history and sex was performed as well.
The association between statin use and clinical outcomes among patients in the IPF cohort was evaluated using Cox proportional hazard regression, and adjusted hazard ratios (HRs) were calculated with corresponding 95% CIs. If a patient started statin therapy before development of the events of interest, further follow-up was censored.
Given that the operational definition of statin use could not provide detailed information about the duration of drug use, additional analysis was performed by dividing statin users according to the duration of drug use, using 5 years as the cut-off point (statin use ≥ 5 years vs. < 5 years vs. nonuse). We compared the effects of statin use ≥ 5 years and statin use < 5 years by including an interaction term in the regression model. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for statistical analyses, and P values < 0.05 for two-tailed tests were considered to be statistically significant.
Data availability
The data that support the findings of this study are available from NHIS database, but there are restrictions applied to the availability of these data, which were used under license for the current study and so are not currently publicly available. Data are however available from the corresponding author upon reasonable request and with permission of NHIS.
Abbreviations
- HMG-CoA:
-
3-hydroxy-3-methylglutaryl coenzyme A
- CI:
-
Confidence interval
- ER:
-
Emergency room
- HR:
-
Hazard ratio
- ICD:
-
International classification of diseases
- ILA:
-
Interstitial lung abnormality
- IPF:
-
Idiopathic pulmonary fibrosis
- NHIS:
-
National health insurance service
- OR:
-
Odds ratio
References
Sgalla, G. et al. Idiopathic pulmonary fibrosis: Pathogenesis and management. Respir. Res. 19, 32 (2018).
Rogliani, P., Calzetta, L., Cavalli, F., Matera, M. G. & Cazzola, M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Pulm. Pharmacol. Ther. 40, 95–103 (2016).
Heukels, P., Moor, C. C., von der Thusen, J. H., Wijsenbeek, M. S. & Kool, M. Inflammation and immunity in IPF pathogenesis and treatment. Respir. Med. 147, 79–91 (2019).
Ruwanpura, S. M., Thomas, B. J. & Bardin, P. G. Pirfenidone: Molecular mechanisms and potential clinical applications in lung disease. Am. J. Respir. Cell Mol. Biol. 62, 413–422 (2020).
Wollin, L., Maillet, I., Quesniaux, V., Holweg, A. & Ryffel, B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J. Pharmacol. Exp. Ther. 349, 209–220 (2014).
Idiopathic Pulmonary Fibrosis Clinical Research N. et al. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 366, 1968–1977 (2012).
Quist-Paulsen, P. Statins and inflammation: An update. Curr. Opin. Cardiol. 25, 399–405 (2010).
De Sadeleer, L. J. et al. Statins: Cause of fibrosis or the opposite? Effect of cardiovascular drugs in idiopathic pulmonary fibrosis. Respir. Med. 176, 106259 (2021).
Kreuter, M. et al. Statin therapy and outcomes in trials of nintedanib in idiopathic pulmonary fibrosis. Respiration 95, 317–326 (2018).
Kreuter, M. et al. Effect of statins on disease-related outcomes in patients with idiopathic pulmonary fibrosis. Thorax 72, 148–153 (2017).
Vedel-Krogh, S., Nielsen, S. F. & Nordestgaard, B. G. Statin use is associated with reduced mortality in patients with interstitial lung disease. PLoS One 10, e0140571 (2015).
Ekstrom, M. & Bornefalk-Hermansson, A. Cardiovascular and antacid treatment and mortality in oxygen-dependent pulmonary fibrosis: A population-based longitudinal study. Respirology 21, 705–711 (2016).
Kim, J. W., Barrett, K., Loke, Y. & Wilson, A. M. The effect of statin therapy on disease-related outcomes in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Respir. Med. Res. 80, 100792 (2021).
Dey, T., Mukherjee, A. & Chakraborty, S. A practical overview of case-control studies in clinical practice. Chest 158, S57–S64 (2020).
Richeldi, L., Collard, H. R. & Jones, M. G. Idiopathic pulmonary fibrosis. Lancet 389, 1941–1952 (2017).
Bai, L. et al. Idiopathic pulmonary fibrosis and diabetes mellitus: A meta-analysis and systematic review. Respir. Res. 22, 175 (2021).
Rangarajan, S. et al. Metformin reverses established lung fibrosis in a bleomycin model. Nat. Med. 24, 1121–1127 (2018).
Park, S. et al. Long-term follow-up of interstitial lung abnormality: Implication in follow-up strategy and risk thresholds. Am. J. Respir. Crit. Care Med. 208, 858–867 (2023).
Hatabu, H. et al. Interstitial lung abnormalities detected incidentally on CT: A position paper from the fleischner society. Lancet Respir. Med. 8, 726–737 (2020).
Hata, A., Schiebler, M. L., Lynch, D. A. & Hatabu, H. Interstitial lung abnormalities: State of the art. Radiology 301, 19–34 (2021).
Duckworth, A. et al. Telomere length and risk of idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease: A mendelian randomisation study. Lancet Respir. Med. 9, 285–294 (2021).
Boccardi, V. et al. A new pleiotropic effect of statins in elderly: Modulation of telomerase activity. FASEB J. 27, 3879–3885 (2013).
Astuti, Y., Wardhana, A., Watkins, J., Wulaningsih, W. & Network, P. R. Cigarette smoking and telomere length: A systematic review of 84 studies and meta-analysis. Environ. Res. 158, 480–489 (2017).
Santos, D. M. et al. Screening for YAP inhibitors identifies statins as modulators of fibrosis. Am. J. Respir Cell Mol. Biol. 62, 479–492 (2020).
Zhu, B., Ma, A. Q., Yang, L. & Dang, X. M. Atorvastatin attenuates bleomycin-induced pulmonary fibrosis via suppressing iNOS expression and the CTGF (CCN2)/ERK signaling pathway. Int. J. Mol. Sci. 14, 24476–24491 (2013).
Watts, K. L., Sampson, E. M., Schultz, G. S. & Spiteri, M. A. Simvastatin inhibits growth factor expression and modulates profibrogenic markers in lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 32, 290–300 (2005).
Jain, M. K. & Ridker, P. M. Anti-inflammatory effects of statins: Clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 4, 977–987 (2005).
Ko, S. J., Choi, S. M., Han, K. D., Lee, C. H. & Lee, J. All-cause mortality of patients with idiopathic pulmonary fibrosis: A nationwide population-based cohort study in Korea. Sci. Rep. 11, 15145 (2021).
Yeh, J. J., Lai, J. N., Lin, C. L., Hsu, C. Y. & Kao, C. H. Time-dependent propensity-matched general population study of the effects of statin use on cancer risk in an interstitial lung disease and pulmonary fibrosis cohort. BMJ Open 11, e047039 (2021).
Petnak, T., Lertjitbanjong, P., Thongprayoon, C. & Moua, T. Impact of antifibrotic therapy on mortality and acute exacerbation in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Chest 160, 1751–1763 (2021).
Pazzagli, L. et al. Methods for time-varying exposure related problems in pharmacoepidemiology: An overview. Pharmacoepidemiol. Drug Saf. 27, 148–160 (2018).
Kuo, C. L., Duan, Y. & Grady, J. Unconditional or conditional logistic regression model for age-matched case-control data?. Front. Public Health 6, 57 (2018).
Pearce, N. Analysis of matched case-control studies. BMJ 352, i969 (2016).
Cheol Seong, S. et al. Data resource profile: The national health information database of the national health insurance service in South Korea. Int. J. Epidemiol. 46, 799–800 (2017).
Seong, S. C. et al. Cohort profile: The national health insurance service-national health screening cohort (NHIS-HEALS) in Korea. BMJ Open 7, e016640 (2017).
Moon, S. W. et al. Longitudinal changes in clinical features, management, and outcomes of idiopathic pulmonary fibrosis. A nationwide cohort study. Ann. Am. Thorac. Soc. 18, 780–787 (2021).
Bae, W. et al. Impact of smoking on the development of idiopathic pulmonary fibrosis: Results from a nationwide population-based cohort study. Thorax 77, 470–476 (2022).
Acknowledgements
We thank the National Health Insurance Service (NHIS) of South Korea for supplying the data for this study.
Author information
Authors and Affiliations
Contributions
SMC is the study lead and guarantor for this paper. JP, CHL, KH, and SMC contributed to conception and design of the study. JP and KH contributed to acquisition, analysis, and interpretation of data. KH performed the main statistical analysis, and JP, CHL, and SMC critically appraised those results. JP wrote the first draft of this paper, and CHL, KH, and SMC revised it critically for important intellectual content. JP, CHL, KH, and SMC had access to final version of this paper and approved it to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, J., Lee, CH., Han, K. et al. Association between statin use and the risk for idiopathic pulmonary fibrosis and its prognosis: a nationwide, population-based study. Sci Rep 14, 7805 (2024). https://doi.org/10.1038/s41598-024-58417-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58417-9
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.