Abstract
This retrospective study aimed to determine the optimal cutoff values of the Dry Eye-Related Quality-of-Life Score (DEQS) questionnaire for diagnosing dry eye disease (DED) and classifying DED severities. Participants completed the DEQS questionnaire, the Japanese version of the Ocular Surface Disease Index (J-OSDI) questionnaire, and DED examinations. DED was diagnosed according to the 2016 Asia Dry Eye Society diagnostic criteria based on DED symptoms (J-OSDI ≥ 13 points) and tear film breakup time ≤ 5 s. Receiver operating characteristic (ROC) analysis was used to calculate the optimal cutoff values of the DEQS summary score for detecting DED and grading its severity. Among 427 patients, 296 (69.3%) and 131 (30.7%) were diagnosed with DED and non-DED, respectively. ROC analysis determined an optimal cutoff value of 15.0 points for DED diagnosis, with 83.5% sensitivity, 87.0% specificity, and an area under the curve of 0.915. The positive and negative predictive values for DEQS ≥ 15.0 points were 93.6% and 69.9%, respectively. DEQS cutoff values of 15.0, 20.0, and 26.8 points could be accepted for severity classification of DED subjective symptoms in clinical use and represent mild, moderate, and severe DED, respectively. Conclusively, the optimal cutoff values of DEQS enable DED detection and subjective symptom severity classification.
Similar content being viewed by others
Introduction
Dry eye disease (DED) is a common ocular surface disorder worldwide1,2. This multifactorial disease can lead to ocular surface damage and severely affect patients’ vision, quality of life, and work productivity3,4. These conditions are mainly caused by the instability of tear film homeostasis, which is the core pathogenesis of DED occurrence5. The assessment of the subjective symptoms of DED and dry eye examinations, primarily the tear film breakup time (TFBUT), constitute the key diagnostic elements of diagnosis criteria, such as the Tear Film and Ocular Surface Society and Asia Dry Eye Society5,6.
Subjective questionnaires continue to be important tools for assessing subjective symptoms of DED7. The Ocular Surface Disease Index (OSDI) questionnaire may be the most widely used in clinical research and screening; however, it does not cover all dry eye symptoms, such as foreign body sensation and health-related quality of life issues7,8,9. In contrast, the Dry Eye-Related Quality-of-Life Score (DEQS) questionnaire, developed in Japan in 2013, considers health-related quality-of-life issues10,11. The validity and reliability of the DEQS have been confirmed to evaluate the multifaceted effects of DED on patients’ daily lives, including ocular symptoms and mental health9,11,12. The DEQS questionnaire showed strong correlations with four subscales (ocular pain, near vision, distance vision, and mental health) of the National Eye Institute Visual Function Questionnaire 256. Additionally, the DEQS was significantly correlated with the Japanese version of the OSDI (J-OSDI) questionnaire, with insignificant score differences13, suggesting that the DEQS questionnaire could be considered equivalent to the J-OSDI. However, different cutoff values of the DEQS summary score have been reported in previous studies9,11, as there is no optimal cutoff value for the DEQS questionnaire14. Additionally, the absence of a clear grading system could affect the classification of subjective symptoms, thereby affecting the treatment of diseases15. Thus, it was necessary to investigate the optimal cutoff values of the DEQS summary score based on subjective symptoms and TFBUT for DED detection and severity classification.
Accordingly, this study aimed to determine the optimal DEQS cutoff values for DED detection and symptom severity categorization by evaluating the sensitivity and specificity of the DEQS based on clinical symptoms and TFBUT. We believe that the DEQS questionnaire can be used more extensively for diagnosing DED in clinical practice, health check-up screening, and online medical services.
Results
Participants’ characteristics
A total of 427 individuals were enrolled; among them, 296 (69.3%) were diagnosed with DED, and 131 (30.7%) were diagnosed with non-DED according to the 2016 Asia Dry Eye Society diagnostic criteria. No significant differences in age and sex were observed between the non-DED and DED groups. The DED group had a significantly higher DEQS summary score and J-OSDI total score and lower TFBUT and maximum blink interval (MBI)16,17 than those in the non-DED group (Supplemental Table S1).
Correlations between DEQS and other clinical assessment findings
We examined the relationship between the DEQS summary score and other clinical assessment findings (J-OSDI, TFBUT, corneal and conjunctival fluorescein staining (CFS), Schirmer I test (SΙT), and MBI) using Pearson’s correlation test. Pearson correlation analysis showed that the DEQS summary score was significantly positively correlated with the J-OSDI total score (P = 0.874) and negatively correlated with the MBI (P = − 0.236) (Table 1).
ROC curves of the DEQS for DED detection
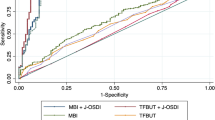
In the receiver operating characteristic (ROC) curve for the sensitivity and specificity of the DEQS, the area under the curve (AUC) calculated based on ROC was 0.915. The optimal cutoff value of the DEQS for DED detection was 15.0 points, which yielded a sensitivity of 83.5% and a specificity of 87.0% (Fig. 1).
Receiver operating characteristic (ROC) analysis of the Dry Eye-Related Quality-of-Life Score for dry eye disease diagnosis. The area under the curve calculated based on the ROC was 0.915. The optimal cutoff value for the Dry Eye-Related Quality-of-Life Score was 15.0 points, which yielded a sensitivity of 83.5% and a specificity of 87.0%.
DEQS cutoff values for DED severity classification via ROC analysis
The DEQS cutoff values for DED severity classification were calculated via ROC analysis corresponding to the J-OSDI score categorization. The optimal cutoff values are listed in Table 2.
Precision rate detected by the DEQS at the cutoff value of 15.0 points
Table 3 shows the precision rates of diagnosis using TFBUT with the DEQS. The positive and negative predictive values for DEQS ≥ 15 points were 93.6% (247/264) and 69.9% (114/163), respectively (Table 3). The sensitivity and specificity were 83.5% (247/296 individuals) and 87.0% (114/131 individuals), respectively (Table 3).
Characteristics of participants detected by DEQS at different cutoff values
The DEQS measurements of participants with different DED severities were compared with those without DED. Based on the DEQS cutoff value of 15 points, the mild DED group (DEQS ≥ 15.0 to < 20.0 points) had significantly higher J-OSDI (P < 0.001) and lower MBI (P < 0.001) than the non-DED group (DEQS < 15 points) (Table 4). Similarly, the moderate DED group (DEQS ≥ 20.0 to < 26.8 points) also showed significantly higher J-OSDI (P < 0.001) and lower MBI (P < 0.001) than the non-DED group (Table 4). A similar result was shown in the severe DED group (DEQS ≥ 26.8 points), which had a higher J-OSDI (P < 0.001) and a lower MBI (P < 0.001) than the non-DED group (Table 4).
Discussion
DED is a highly prevalent chronic condition of the ocular surface. The DEQS questionnaire correlates well with the severity of subjective symptoms and effects of DED on daily life18. However, the DEQS questionnaire has no optimal cutoff values for DED diagnosis and severity classification, which limits its use in the clinical setting. Thus, we aimed to detect DED and categorize the severity of the subjective symptoms of DED with optimal DEQS cutoff values using a hospital-based, cross-sectional, observational method. This study yielded a DEQS cutoff value of 15.0 points for DED diagnosis. Further, DEQS cutoff values of 15.0, 20.0, and 26.8 points could be adopted for the severity grading of subjective symptoms in clinical use. Notably, the DEQS with optimal cutoff values could be used in clinical studies for DED diagnosis and severity classification.
Different cutoff values of the DEQS have been reported in previous studies9,11. In a study conducted in Thailand, DED was suspected if the DEQS cutoff value was > 18.33 (AUC, 0.897; sensitivity, 90.0%; specificity, 76.7%)9. Their research adopted the following diagnostic criteria: ocular symptoms (OSDI ≥ 13 points) and tear film abnormality (TFBUT ≤ 5 s or SIT with anesthesia < 5 mm)9. Another study using a combination of the DEQS questionnaire and strip meniscometry score reported an optimal cutoff value of 15.0 points for the DEQS questionnaire and a strip meniscometry score < 5 mm (AUC, 0.904; sensitivity, 79.4%; specificity, 90.6%)11. In the current study, we explored the optimal DEQS cutoff value for DED diagnosis, which was DEQS ≥ 15.0 points combined with TFBUT ≤ 5 s (AUC, 0.915; sensitivity, 83.5%; specificity, 87.0%). Additionally, we discovered that the positive and negative predictive values of DEQS ≥ 15.0 points for predicting DED were 93.6% and 69.9%, respectively. In this study, we observed a limited number of false positives and false negatives due to score differences between DEQS and J-OSDI. DEQS includes questions related to depressive symptoms and eye problems associated with prolonged mobile phone screen use, whereas J-OSDI addresses environmental factors and the impact of DED on night driving. These differences in the questionnaire items that contribute to the scoring systems may have influenced the score variations between DEQS and J-OSDI13. However, despite these differences, the accuracy of DED diagnosis using a DEQS cutoff of ≥ 15 points remained consistently high. Hence, a DEQS questionnaire cutoff value of 15.0 points was deemed suitable for DED diagnosis.
To our knowledge, this is the first study to report the optimal cutoff values of the DEQS for the severity classification of subjective symptoms of DED. DEQS cutoff values of 15.0, 20.0, and 26.8 points in our study corresponded to the respective J-OSDI total scores of 13.0, 23.0, and 33.0 points, which are the suggested cutoff scores for mild, moderate, and severe degrees of DED on the J-OSDI scale19. Among the four groups classified by the above-mentioned optimal DEQS cutoff values, MBI values displayed a gradual downward trend (13.3 ± 7.4, 10.6 ± 6.8, 10.3 ± 6.6, and 10.0 ± 6.5 in DEQS < 15, ≥ 15.0 to < 20.0, ≥ 20.0 to < 26.8, and ≥ 26.8 groups, respectively). Hence, the descending tendency observed in the MBI values could show the classification effect of the DEQS optimal cutoff values proposed in this study. Notably, the DEQS cutoff value of 25.0 points is very close to the DEQS cutoff value of 26.8 points, as determined by the Youden index (0.741 vs. 0.747) (Supplemental Table S2). Therefore, considering the ease of use in a clinical setting, DEQS values of 15.0, 20.0, and 25.0 points could be proposed as the optimal cutoff values for DED severity stratification. Categorizing the severity of DED subjective symptoms is beneficial for patients’ self-management of DED and for clinicians to diagnose and treat DED more efficiently. As a result, medical resources can be used more effectively.
Here, when the DEQS cutoff value was set as 15.0 points, the DEQS ≥ 15.0 to < 20.0 group had a significantly lower MBI (10.6 ± 6.8 vs. 13.3 ± 7.4 seconds, P < .001) than the DEQS < 15.0 group. Similar results were also presented in the DEQS ≥ 20.0 to < 26.8 (10.3 ± 6.6 vs. 13.3 ± 7.4 seconds, P < .001) and DEQS ≥ 26.8 (10.0 ± 6.5 vs. 13.3 ± 7.4 seconds, P < .001) groups, with lower MBI than that of the DEQS < 15.0 group. MBI is a simple method for DED screening and was positively correlated with TFBUT in a previous study16. In our study, MBI was significantly associated with TFBUT. Recent epidemiological surveys have shown that shorter TFBUT is the most common manifestation of DED in clinical practice20,21,22. However, no significant difference was discovered in the TFBUT value between the DEQS < 15.0 group and the other three groups due to low TFBUT values. DED could be diagnosed using the DEQS with an optimal cutoff value of 15.0 points based on the MBI differences between the two groups. Thus, a DEQS cutoff value of 15.0 points is optimal for identifying DED using the MBI screening method.
The DEQS questionnaire possesses good internal consistency, test–retest reliability, discriminant validity, and responsiveness to change9,10. The optimal cutoff values for the DEQS obtained through our hospital-based, cross-sectional study, combined with a TFBUT value ≤ 5 s, enabled DED diagnosis and symptom severity classification with high sensitivity, specificity, and AUC. Additionally, the DEQS optimal cutoff values corresponded well with the proposed J-OSDI cutoff values for classifying DED severity. Therefore, considering the comprehensive nature of the DEQS compared with that of traditional dry eye-related questionnaires, the DEQS with the proposed optimal cutoff values for DED detection and severity classification may be better suited for wider implementation. Early diagnosis, together with timely treatment, could relieve dry eye symptoms and improve the quality of life of patients with DED, reducing the socioeconomic burden on society as a whole.
This study had some limitations. First, the data were collected using a Japanese questionnaire and exclusively from a single hospital in Tokyo, Japan. This could introduce selection bias, and as a result, the findings from this study may not be readily applicable to broader Asian populations. Second, the participants were older (60.3 ± 16.0 years), and there were more female participants, possibly due to the higher DED prevalence in older and female populations. Finally, the parameters of dry eye examinations, including TFBUT, SIT, and CFS, did not exhibit significant differences between these groups (Supplemental Table S3). Since our study only included individuals whose dry eye subjective symptoms had improved with eye drops and classified them into the non-DED group, these population characteristics had a potentially negative impact on the recorded dry eye test values in our study23. However, the MBI values showed a gradual downward trend in these groups, which could still reflect the DEQS capability to classify DED severity.
In conclusion, the present study illustrated the optimal cutoff values of the DEQS for DED detection and DED severity categorization in clinical activities. The combination of TFBUT and the DEQS with an optimal cutoff value for DED diagnosis generated high sensitivity and specificity. The optimal cutoff values of the DEQS for DED severity classification based on the J-OSDI standard also had high sensitivity and specificity via ROC analysis. Thus, the current study demonstrates the potency and feasibility of the DEQS for DED identification and severity classification in clinical diagnosis and health examination screening.
Methods
Study design and population
This study was a retrospective, hospital-based, cross-sectional, observational study. Outpatients who visited the Department of Ophthalmology at Juntendo University Hospital in Tokyo, Japan, were included in the study between September 2017 and September 2021. The enrolled patients comprised individuals with DED and other concomitant ocular diseases, all of whom underwent comprehensive ophthalmological assessments, including dry eye examinations. DED was diagnosed according to the Asia Dry Eye Society 2016 diagnostic criteria based on the presence of DED symptoms (J-OSDI ≥ 13 points as positive) and a TFBUT ≤ 5 s5. Patients with a history of eyelid disorder, ptosis, mental disease, Parkinson’s disease, severe ocular allergy diseases, those immediately after surgery, or any other disease that could affect blinking, as per previous research16,17, were excluded. The participants completed various assessments and ophthalmic examinations, including the DEQS questionnaire, J-OSDI questionnaire, slit-lamp microscopy, TFBUT, CFS, SIT, and MBI16.
Ethical approval
This study was approved by the Independent Ethics Committee of Juntendo University Faculty of Medicine (approval number: E22-0365-H01) and was performed in accordance with the Declaration of Helsinki. The requirement for written informed consent was waived owing to the retrospective nature of the study by the Independent Ethics Committee of Juntendo University Faculty of Medicine; thus, the study was carried out using the opt-out method on our hospital website.
DEQS questionnaire
The DEQS questionnaire primarily assesses the impact of DED on the patients’ quality of life10. It consists of 15 items that evaluate DED symptoms and how they have affected the subjects’ daily lives over the past week. All items in the DEQS questionnaire were divided into two sections: six questions regarding bothersome ocular symptoms and nine questions regarding the impact of DED on daily life. Columns A and B present the frequency and severity of each question, respectively. In column A, respondents provided a rating of the frequency of each symptom using a 5-point scale, ranging from “none of the time” (0 points) to “all the time” (4 points). A frequency score of 1–4 prompted the interviewee to move to column B, where they rated the degree of severity on a 4-point scale. Consequently, the DEQS score, ranging from 0 to 100, was calculated using the following formula: (sum of the degree scores for all questions answered) × 25/(total number of questions answered), with higher scores indicating increased severity of DED symptoms and a greater impact on daily life.
J-OSDI questionnaire
The J-OSDI questionnaire is a translated Japanese version of the OSDI24. The reliability and validity of the DED diagnosis have been demonstrated in our previous studies24,25. The J-OSDI contains three subscales of 12 questions in total: ocular symptoms (three questions), vision-related functions (six questions), and environmental triggers (three questions). The participants were evaluated via graded symptoms on a 5-point scale from 0 points (none of the time) to 4 points (all the time). The J-OSDI total score, ranging from 0 to 100 points, was calculated by multiplying the sum score of all questions answered by 25 and dividing it by the total number of questions answered (N/A is selected when the question is not applicable). According to the J-OSDI total score, patients were stratified into four subgroups: normal (score, 0–12), mild (score, 13–22), moderate (score, 23–32), and severe (score, 33–100) symptoms19,24. The J-OSDI total score was positively associated with DED severity and impact on activities of daily living.
Ocular examination procedures and clinical assessments
TFBUT was measured according to a standard procedure6. Ocular surface damage should be avoided, and the effect on tear volume and TFBUT should be minimized; therefore, fluorescein should be instilled at the outer canthus after removing excess saline on the strip. The participants were instructed to blink thrice to ensure satisfactory mixing of the dye with tears. The period between the last blink and the onset of the first dark spot on the cornea was recorded using a stopwatch. The mean values of three measurements were used. A cutoff value of TFBUT ≤ 5 s was used to diagnose DED5; the eye with the lower TFBUT value was used in the current study.
CFS was categorized based on the van Bijsterveld grading system26, and the ocular surface was divided into three regions: the nasal bulbar conjunctiva, the temporal bulbar conjunctiva, and the cornea. Each area was assessed on a scale of 0–3, with 0 indicating no staining and 3 indicating confluent staining; the maximum possible score was 9. The CFS assessment was conducted using reference diagrams from the van Bijsterveld grading system, as previously described in studies27,28,29.
MBI was defined as the length of time that the participants could keep their eyes open before blinking during each test16,17. MBI was calculated twice using a stopwatch under slit-lamp microscopy with the light off to protect the patient from glare. If MBI exceeded 30 s, it was recorded as 30.
After completion of all other examinations, SIT was performed without topical anesthesia. The strips for SIT were placed in the outer third of the temporal lower conjunctival fornix for 5 min. The strips were then removed, and the length of the wet filter paper (mm) was noted.
Statistical analysis
The unpaired t-test was used for continuous variables, and the χ2 test was used for categorical variables. Pearson correlation coefficients were estimated for the DEQS summary score, J-OSDI total score, TFBUT, CFS, MBI, and SIT.
The ROC curve was created by calculating the sensitivity and specificity to determine the optimal cutoff values of the DEQS summary score for detecting DED and establishing normal, mild, moderate, and severe DED severity categories. The optimal cutoff value for the DEQS was determined when the Youden index, which is the sum of sensitivity and specificity minus one, was maximized30. The accuracy of DED detection using the calculated cutoff value of the DEQS was evaluated in terms of sensitivity, specificity, positive predictive value, and negative predictive value. Participants’ characteristics across DED severity categories, classified using the DEQS cutoff values, were compared to evaluate their validity.
Data are shown as the mean ± standard deviation or proportions (percentages). P values < 0.05 were considered statistically significant. Statistical analysis was performed using Stata version 17.1 (StataCorp, College Station, TX, USA).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
References
Craig, J. P. et al. TFOS DEWS II definition and classification report. Ocul. Surf. 15, 276–283. https://doi.org/10.1016/j.jtos.2017.05.008 (2017).
Wolffsohn, J. S. et al. TFOS Lifestyle: Impact of the digital environment on the ocular surface. Ocul. Surf. 28, 213–252. https://doi.org/10.1016/j.jtos.2023.04.004 (2023).
Stapleton, F. et al. TFOS DEWS II epidemiology report. Ocul. Surf. 15, 334–365. https://doi.org/10.1016/j.jtos.2017.05.003 (2017).
Inomata, T. et al. Smartphone-based digital phenotyping for dry eye toward P4 medicine: A crowdsourced cross-sectional study. NPJ Digit. Med. 4, 171. https://doi.org/10.1038/s41746-021-00540-2 (2021).
Tsubota, K. et al. New perspectives on dry eye definition and diagnosis: A consensus report by the Asia Dry Eye Society. Ocul. Surf. 15, 65–76. https://doi.org/10.1016/j.jtos.2016.09.003 (2017).
Wolffsohn, J. S. et al. TFOS DEWS II diagnostic methodology report. Ocul. Surf. 15, 539–574. https://doi.org/10.1016/j.jtos.2017.05.001 (2017).
Okumura, Y. et al. A review of dry eye questionnaires: Measuring patient-reported outcomes and health-related quality of life. Diagnostics (Basel) 10, 559. https://doi.org/10.3390/diagnostics10080559 (2020).
Abetz, L. et al. Development and validation of the impact of dry eye on everyday life (IDEEL) questionnaire, a patient-reported outcomes (PRO) measure for the assessment of the burden of dry eye on patients. Health Qual. Life Outcomes 9, 111. https://doi.org/10.1186/1477-7525-9-111 (2011).
Tananuvat, N., Tansanguan, S., Wongpakaran, N. & Wongpakaran, T. Reliability, validity, and responsiveness of the Thai version of the dry eye-related quality-of-life score questionnaire. PLoS One 17, e0271228. https://doi.org/10.1371/journal.pone.0271228 (2022).
Sakane, Y. et al. Development and validation of the dry eye-related quality-of-life score questionnaire. JAMA Ophthalmol. 131, 1331–1338. https://doi.org/10.1001/jamaophthalmol.2013.4503 (2013).
Ishikawa, S., Takeuchi, M. & Kato, N. The combination of strip meniscometry and dry eye-related quality-of-life score is useful for dry eye screening during health checkup: Cross-sectional study. Medicine (Baltimore) 97, e12969. https://doi.org/10.1097/md.0000000000012969 (2018).
Shigeyasu, C. et al. Quality of life measures and health utility values among dry eye subgroups. Health Qual. Life Outcomes 16, 170. https://doi.org/10.1186/s12955-018-0999-3 (2018).
Inomata, T. et al. Comparing the Japanese version of the ocular surface disease index and dry eye-related quality-of-life score for dry eye symptom assessment. Diagnostics (Basel) 10, 203. https://doi.org/10.3390/diagnostics10040203 (2020).
Habibzadeh, F., Habibzadeh, P. & Yadollahie, M. On determining the most appropriate test cut-off value: The case of tests with continuous results. Biochem. Med. (Zagreb) 26, 297–307. https://doi.org/10.11613/bm.2016.034 (2016).
Olde Hartman, T. C. et al. What do guidelines and systematic reviews tell us about the management of medically unexplained symptoms in primary care?. BJGP Open https://doi.org/10.3399/bjgpopen17X101061 (2017).
Inomata, T. et al. Maximum blink interval is associated with tear film breakup time: A new simple, screening test for dry eye disease. Sci. Rep. 8, 13443. https://doi.org/10.1038/s41598-018-31814-7 (2018).
Hirosawa, K. et al. Diagnostic ability of maximum blink interval together with Japanese version of ocular surface disease index score for dry eye disease. Sci. Rep. 10, 18106. https://doi.org/10.1038/s41598-020-75193-4 (2020).
Yazdani-Ibn-Taz, M. K. et al. Patient-reported severity of dry eye and quality of life in diabetes. Clin. Ophthalmol. 13, 217–224. https://doi.org/10.2147/opth.S184173 (2019).
Schiffman, R. M., Christianson, M. D., Jacobsen, G., Hirsch, J. D. & Reis, B. L. Reliability and validity of the ocular surface disease index. Arch. Ophthalmol. 118, 615–621. https://doi.org/10.1001/archopht.118.5.615 (2000).
Tong, L., Chaurasia, S. S., Mehta, J. S. & Beuerman, R. W. Screening for meibomian gland disease: Its relation to dry eye subtypes and symptoms in a tertiary referral clinic in Singapore. Invest. Ophthalmol. Vis. Sci. 51, 3449–3454. https://doi.org/10.1167/iovs.09-4445 (2010).
Uchino, M. et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: The Osaka study. Am. J. Ophthalmol. 156, 759–766. https://doi.org/10.1016/j.ajo.2013.05.040 (2013).
Yokoi, N. et al. Importance of tear film instability in dry eye disease in office workers using visual display terminals: The Osaka study. Am. J. Ophthalmol. 159, 748–754. https://doi.org/10.1016/j.ajo.2014.12.019 (2015).
Qian, L. & Wei, W. Identified risk factors for dry eye syndrome: A systematic review and meta-analysis. PLoS One 17, e0271267. https://doi.org/10.1371/journal.pone.0271267 (2022).
Midorikawa-Inomata, A. et al. Reliability and validity of the Japanese version of the ocular surface disease index for dry eye disease. BMJ Open 9, e033940. https://doi.org/10.1136/bmjopen-2019-033940 (2019).
Okumura, Y. et al. DryEyeRhythm: A reliable and valid smartphone application for the diagnosis assistance of dry eye. Ocul. Surf. 25, 19–25. https://doi.org/10.1016/j.jtos.2022.04.005 (2022).
van Bijsterveld, O. P. Diagnostic tests in the Sicca syndrome. Arch. Ophthalmol. 82, 10–14. https://doi.org/10.1001/archopht.1969.00990020012003 (1969).
van Bijsterveld, O. P. & van Hemel, O. L. Sucralfate and sodium sucrose sulphate in the treatment of superficial corneal disease in keratoconjunctivitis sicca. Acta Ophthalmol. (Copenh) 70, 518–521. https://doi.org/10.1111/j.1755-3768.1992.tb02123.x (1992).
Rasmussen, A. et al. Comparison of the American–European Consensus Group Sjogren’s syndrome classification criteria to newly proposed American College of Rheumatology criteria in a large, carefully characterised sicca cohort. Ann. Rheum. Dis. 73, 31–38. https://doi.org/10.1136/annrheumdis-2013-203845 (2014).
Doughty, M. J. Rose bengal staining as an assessment of ocular surface damage and recovery in dry eye disease—A review. Contact Lens Anterior Eye 36, 272–280. https://doi.org/10.1016/j.clae.2013.07.008 (2013).
Chen, F., Xue, Y., Tan, M. T. & Chen, P. Efficient statistical tests to compare Youden index: Accounting for contingency correlation. Stat. Med. 34, 1560–1576. https://doi.org/10.1002/sim.6432 (2015).
Acknowledgements
The authors thank the nurses and orthoptists at the Juntendo University Faculty of Medicine, Department of Ophthalmology, for collecting and measuring data for the DED diagnosis. In addition, this research was supported by JSPS KAKENHI (grant numbers 20KK0207 to T.I.; 21K17311 and 23K16364 to A.M.I.; 22K16983 to A.E.), Takeda Science Foundation (T.I.), and the Ichiro Kanehara Foundation (T.I.).
Author information
Authors and Affiliations
Contributions
Conceptualization, research design, validation, investigation, T.I.; data analysis and writing—original draft preparation, X.Z. and K.N.; data collection and analysis, Y.O., A.M.I., A.E., Kei.F., M.M. H.T., Ken.F., Y.A.; language modification, A.Y.; supervision, S.N., H.K., A.M.; Writing—reviewing and editing, all authors. X.Z. and K.N. contributed equally as co-first authors.
Corresponding author
Ethics declarations
Competing interests
K.N., Y.O., and A.M.I. received personal fees from InnoJin, Inc., outside the submitted work. S.N. received grants from Kowa Co., Ltd., Mitsubishi Tanabe Pharma Corp., Alcon Japan, Ltd., Santen Pharmaceutical Co., Ltd., Machida Endoscope Co., Ltd., Wakamoto Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., Senju Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Chugai Pharmaceutical Co., Ltd., Hoya Corp., and Novartis Pharma K.K. T.I. received grants from Johnson & Johnson Vision Care, Inc., SEED Co., Ltd., Novartis Pharma K.K., and Kowa Company, Ltd., outside the submitted work, as well as personal fees from Santen Pharmaceutical Co., Ltd. and InnoJin, Inc. The remaining authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zou, X., Nagino, K., Okumura, Y. et al. Optimal cutoff value of the dry eye-related quality-of-life score for diagnosing dry eye disease. Sci Rep 14, 4623 (2024). https://doi.org/10.1038/s41598-024-55358-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55358-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.