Abstract
This study aimed to examine the impact of term LBW on short-term neonatal and long-term neurodevelopmental outcomes in children 5–7 years of age. This is a population-based cohort study that merged national data from the Korea National Health Insurance claims and National Health Screening Program for Infants and Children. The participants were women who gave birth at a gestational age of ≥ 37 weeks between 2013 and 2015 in the Republic of Korea, and were tracked during 2020 for the neurodevelopmental surveillance of their children. Among 830,806 women who gave birth during the study period, 31,700 (3.8%) of their babies weighed less than 2500 g. By Cox proportional hazard analysis, children aged 5–7 years who had LBW were associated with any developmental, motor developmental delay, cognitive developmental delay, autism spectrum, attention deficit hyperactivity disorders, and epileptic and febrile seizures.Children born with term LBW were more vulnerable to neurodevelopmental disorders at 5–7 years of age than those with normal and large birth weights. This study further substantiates counseling parents regarding the long-term outcomes of children being born underweight.
Similar content being viewed by others
Introduction
Birth weight indicates infant well-being and is a key factor in infant health policy. According to the World Health Organization, low birth weight (LBW) is defined as a birth weight of less than 2500 g irrespective of gestational age while fetal growth restriction(FGR) or small for gestational age (SGA) refers to estimated fetal weight or birthweight below the 10th percentile for gestational age1. The estimated incidence of LBW is more than 20 million infants worldwide2,3. The prevalence of LBW varies in low- and middle-income countries and could be as low as 2–3% or as high as 30%2,4. We recently conducted a study in collaboration with over 20 countries, examined the global pattern of LBW in an exclusive manner and found a varied distribution, with the highest incidence rate observed in Southwest Asia5. The main causes of LBW are premature birth and poor fetal growth. Yet mostly unidentified, chromosome abnormality, infection and placental dysfunction are major causes of poor fetal growth. Globally, infants born with LBW are susceptible to short- and long-term adverse health outcomes including neurodevelopmental disorders6,7. Studies have been inconsistent and inconclusive, but they emphasized that LBW children have a higher risk of developmental delay, lower cognitive and motor function, and more behavioral problems than normal birth weight children8,9,10,11,12. LBW adds to the public health burden13.
Understanding neurodevelopment in LBW children enables early and timely intervention for developmental delay, which could accelerate and improve health outcomes. Responsive stimulation during early life is crucial for later cognitive development in children14. Discussions on prematurity and LBW have been widely conducted, and while not fully established, causal relationships have been acknowledged. However, there is limited research on the association between the LBW of term-born infants and developmental outcomes. This population cohort study aimed to determine developmental outcomes including developmental delay, behavioral problems, and cognitive and motor performance in LBW infants compared to normal and large birth weight infants up to 7 years of age in South Korea.
Materials and methods
Data characteristics
This study was conducted by merging national data from the Korea National Health Insurance (KNHI) claims, National Health Screening Examination (NHSE), and National Health Screening Program for Infants and Children (NHSP-IC). In Korea, 97% of the population is enrolled in the KNHI program, and its claims database contains all their claims information. Therefore, this centralized database contains comprehensive information on diseases and their treatments except for non-insurance procedures. Using this database, we identified all pregnant women who delivered between January 1, 2013, and December 31, 2015. Subsequent developmental delay, motor developmental delay, cognitive developmental delay, autism spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD), and epileptic and febrile seizures in their children up to 5–7 years were tracked until December 31, 2020. The KNHI system provides an NHSP-IC linked to maternal data for all neonates. Key components of the NHSP-IC include a health examination of the children, assessment of their gestational age at delivery, and measurement of birth weight. This study was approved by the Institutional Review Board of the Korea University Medical Center (No. 2023GR0196) which waved the requirement for informed consent for the following reason. All information was provided for the study after it had been anonymized; therefore, informed consent was not obtained from the participants. All methods were performed in accordance with the declaration of Helsinki.
Study population
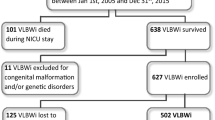
A flowchart of patient enrollment is shown in Fig. 1. Birth weight was categorized as LBW < 2500 g, normal birth weight 2500–3999 g, and large birth weight ≥ 4000 g. Women who had a gestational age of ≥ 37 weeks were included. Among these, women who had multiple pregnancies, preterm births, fetal malformations, syndromes and other abnormalities allocated Q code in the International Classification of Disease-10th Revision (ICD-10), women whose children did not undergo the NHSP-IC, and missing values were excluded. Perinatal factors were obtained on maternal characteristics (age, type of delivery, pregnancy-induced hypertension, gestational diabetes (GDM), hypertension and diabetes mellitus (DM) diagnosed before pregnancy) and infant characteristics (sex and birthweight) using the KNHI claims dataset. In addition, the medical issues of the infants including transient tachypnea, respiratory distress syndrome (RDS), necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), bronchopulmonary dysplasia (BPD) and birth asphyxia were identified. Underlying maternal and neonatal diseases were identified according to the ICD-10 codes.
Short-term outcomes of infants
Using the KNHI claims dataset, transient tachypnea, RDS, NEC, IVH, BPD and birth asphyxia were identified by principal or secondary diagnosis based on the codes of the ICD-10.
Follow-up neurodevelopmental outcomes up to 5–7 years
Any developmental delay, motor developmental delay, cognitive developmental delay, ASD, ADHD, tics and stereotypic behavior, and epileptic and febrile seizures were identified by principal or secondary diagnosis based on the ICD-10 codes (Table 1).
Statistical analysis
Continuous and categorical variables were expressed as mean ± standard deviation (SD) and percentages. Clinical and biochemical characteristics were compared among the groups using the t-test or one-way analysis of variance (ANOVA) for continuous variables and the χ2 test for categorical variables. The cumulative incidence of developmental delay, ASD, ADHD, tics and stereotypic behavior, and seizure and epileptic disorder was estimated using the Kaplan–Meier method and compared using the log-rank test. Cox proportional hazards models were used to estimate the adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the rates of transient tachypnea, RDS, NEC, IVH, and BPD and for the development of developmental delay, ASD, ADHD, tics and stereotypic behavior, and seizure and epileptic disorder. Confounding factors adjusted were maternal age, undergoing cesarean section, pregnancy-induced hypertension, gestational diabetes, overt DM, hypertension before pregnancy, and the sex of the baby. Neonatal short-term complications were adjusted for analysis. Based on univariate analysis, variables with p-values < 0.2 were included in the Cox proportional hazards models. All tests were two-sided values, and p-values < 0.05 were considered statistically significant. Statistical analyses were performed using SAS for Windows, version 9.4 (SAS Inc., Cary, NC, USA).
Results
Characteristics of participants
The obstetric characteristics of the participants are presented in Table 2. Women with LBW and large birth weight babies were older and had a higher prevalence of undergoing cesarean sections than those with normal birth weight babies. The rates of pregnancy-induced hypertension and hypertension before pregnancy were significantly higher in women with LBW babies than in those with large or normal birth weight babies. Women with large birth weight babies had significantly higher rates of gestational and overt diabetes than those with normal or LBW babies. Male sex was more prevalent in large birth weight babies than normal or LBW babies.
Short-term complications in neonates
Cox proportional hazards models were used to estimate the adjusted HRs (aHRs) and 95% CIs for the development of short-term neonatal outcomes related to birth weight after adjustment for confounding factors (Table 3). Neonates with LBW had significantly higher rates of transient tachypnea (aHR 2.34, 95% CI 2.19–2.49), RDS (aHR 8.81, 95% CI 8.37–9.27), NEC (aHR 10.61, 95% CI 7.69–14.6), IVH (aHR 20.99, 95% CI 17.58–25.1), BPD (aHR 115.2, 95% CI 84.1–157.9), and birth asphyxia (aHR 3.52, 95% CI 2.82–4.39) than those with normal birth weight. Neonates with large birthweight had more transient tachypnea (aHR 1.54, 95% CI 1.43–1.66), IVH (aHR 1.62, 95% CI 1.02–2.59), and BPD (aHR 3.79, 95% CI 1.79–8.03) than those with normal birth weight.
Neurodevelopmental outcome stratified by birth weight
The neurodevelopmental outcomes related to the birth weight of the neonates were estimated using Cox proportional hazards models after adjustment for confounding factors (Table 4). LBW was associated with any developmental delay (aHR 1.36, 95% CI 1.29–1.44), motor developmental delay (aHR 1.31, 95% CI 1.22–1.42), cognitive developmental delay (aHR 1.43, 95% CI 1.33–1.54), ASD (aHR 1.76, 95% CI 1.59–1.95), ADHD (aHR 1.30, 95% CI 1.17–1.46), and epileptic and febrile seizures (aHR 1.14, 95% CI 1.10–1.18). There was no difference in neurodevelopmental outcomes between neonates with large and normal birth weights. Figure 2 shows Kaplan–Meier curves for the cumulative incidence of neurodevelopmental outcomes among the low, normal, and large birth weight groups. Up to 7 years of age, the cumulative incidences of motor, cognitive, and any developmental delays, in addition to ASD, ADHD, and epileptic and febrile seizures were significantly higher in the LBW group than in normal and large birth weight groups.
Discussion
This study examined the short- and long-term outcomes, including neurodevelopmental disorders, of babies with low, normal, and large birth weights using national population data. Children with LBW were not only susceptible to short-term complications but also suffered increasingly from motor, cognitive, and any developmental delays, ASD, ADHD, and epileptic and febrile seizures up to the age of 7.
LBW is a risk factor for neonatal morbidity and mortality, overall health, and developmental disorders15. However, previous studies have predominantly focused on the impact of LBW due to preterm birth, indicating that prematurity itself is a major cause of developmental disorders16, and research on LBW infants born at term has focused on IQ, learning, and behavior17,18. In addition, the definitions of small for gestational age (SGA) or fetal growth restriction, children age at assessment and outcome variables were varied with or without deficits in cognitive and learning abilities, and occurrence of attention problems19. Few studies have targeted the Asian population in this aspect.
Consistent with our findings, a Norwegian population study recently demonstrated a highly significant dose–response association between birthweight and cerebral palsy, vision/hearing disability, intellectual impairment, schizophrenia, epilepsy, ASD, and behavioral disorders such as ADHD20. Sacchi et al. performed a meta-analysis of 2230 children born at term who had intrauterine growth restriction or were SGA and demonstrated that they had lower cognitive scores than those who were appropriate for gestational age15. However, the other studies demonstrated no differences or mixed results in developmental outcomes, including school performance, attention problems, and psychological symptoms21,22,23. This could be attributed to methodological limitations due to multiple contributing factors, such as different ethnic backgrounds, social interactions, and economic aspects. Given the impact of ethnic differences on LBW infants, our study’s specific focus on the Asian population is of particular importance.
Poor growth, maternal malnutrition, poverty, stress, infections such as malaria and HIV, diarrhea, environmental toxins, psychosocial factors such as learning opportunities, caregiver interaction, violence, and maternal depression can contribute to the deterioration child brain functioning24,25. However, the exact physiological mechanisms behind LBW and its effects on development are not yet fully understood due to the complexity of the causal pathways involved. One possible theory is related to fetal programming. Abnormal fetal growth is a sign of substantial alterations in fetal programming. The fetus adapts and survives in the uterus by slowing its growth. Defective placentation is a phenomenon that results in poor fetal growth, and may cause hypoxemia, inflammation, undernutrition, and endocrine dysregulation, which could impair normal brain development25,26. It could also lead to chronic hypoxia, which causes poor growth and consequently cerebral injury, especially in the primary sensory and forebrain motor systems, resulting in cognitive, motor, and attentional deficits27. Barker's theory supports the concept of fetal programming, indicating that individuals born under conditions of inadequate nutrition during pregnancy face a higher risk of ischemic heart disease and mortality as adults28. This evidence has been further expanded in recent years, suggesting that children born in suboptimal conditions in the uterus may experience increased health issues.
Cerebral microstructural and metabolic changes were evident in fetal brain magnetic resonance imaging of LBW infants, and this might cause abnormal neurodevelopment29. Placental dysfunction, a situation which the fetus has a limited transport of nutrients and oxygen also explains the association between LBW and ASD. Consistent with our finding that the ASD rate was significantly increased in LBW infants, a meta-analysis including more than 8 million participants demonstrated a significant association between infants who were SGA and the risk of ASD30.
Morbidities associated with LBW such as RDS and BPD may cause poor neurodevelopment31,32. Hypoxia caused by short-term respiratory problems in the neonatal period can interfere with normal brain development. As demonstrated by our data, LBW infants are more likely to suffer from complications during the neonatal period owing to the suboptimal oxygen levels that can damage their rapidly growing neurons and prevent them from forming new connections. Despite our analysis adjusting for neonatal complications, there was still an increased rate of developmental delay in LBW infants.
There have been limited studies on the association between LBW and tics and stereotypic disorders, which are highly complex and multifactorial in etiology. A study on the Korean population reported associations between tics and perinatal factors and revealed no association with LBW33 which is consistent with our findings. However the age of onset of tics and stereotypic disorders is usually 4–6 years, and since our study included children aged 5–7 years, there are limitations in establishing an association between LBW and tics and stereotypic disorders.
Our study findings on the relationship between LBW infants born on term and developmental outcomes prompt the identification of risk factors and early intervention. Several modifiable factors in the early environment may have long-term effects on health and cognitive function. In a British study, the learning environment (parental reading and interest in education) influenced cognitive development independent of birth weight and social background34. A prospective study suggested that stimulation at home may improve neurodevelopment in LBW children, especially during the early period35. According to a study on Korean adopted children, developmental outcomes may differ within the first 2 years depending on environmental factors and nutritional supply alone36. This emphasizes the significance of the early identification of children who are at risk of cognitive and motor deficits, closely monitoring their development and intervening promptly and effectively to enhance their developmental abilities.
Parents typically do not consider their LBW infants born on term to be at a risk of developmental disadvantages, and their postnatal care is underestimated. Therefore, it is crucial for healthcare professionals to educate parents about the importance of early screening and evaluation of these infants to ensure their healthy development.
This study boasts several notable strengths, primarily deriving from a population-based cohort, which offers the advantage of comprehensive and extended follow-up. Furthermore, to mitigate potential sources of bias, the study meticulously identified and controlled for numerous underlying risk factors pertaining to perinatal characteristics. Nonetheless, it is imperative to approach our findings with a degree of caution, recognizing several limitations. First and foremost, the neurodevelopmental outcomes of the children under scrutiny in this research were determined based on ICD-10 codes extracted from insurance claims data. Consequently, questions regarding the reliability and accuracy of these diagnoses within this database may arise detailed diagnostic methods used for developmental delay have not been reviewed. However, a recent study showed a 78.1–88.7% sensitivity and 100% specificity when comparing the diagnoses obtained from insurance claims data with the verified diagnoses documented in patient medical records37. Furthermore we encountered limitations in accessing essential information regarding influential factors of growth and neurodevelopment, such as socioeconomic status, cognitive and motor stimulation during early life, parent BMI and education, social environment, and nutritional status after birth. Prospective studies considering these factors are needed to further investigate the association between birth weight and neurodevelopmental outcomes.
In summary, the findings of this study show that developmental delay, ASD, ADHD, and epileptic and febrile seizures were increased in LBW infants born at term. These findings support the notion that LBW infants born at term need timely evaluation of their neurodevelopment because early detection and intervention are key to improved quality of life. The findings of this study need to be confirmed in a prospective study. Furthermore, studies on the mechanisms involved in neurodevelopment associated with LBW should be conducted.
Data availability
The dataset generated during and analyzed during the current study are not publicly available due to dataset owned by government but are available from the corresponding author on reasonable request.
Abbreviations
- ADHD:
-
Attention deficit hyperactivity disorder
- ASD:
-
Autism spectrum disorder
- BPD:
-
Bronchopulmonary dysplasia
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- IVH:
-
Intraventricular hemorrhage
- NEC:
-
Necrotizing enterocolitis
- NHSP-IC:
-
National Health Screening Program for Infants and Childre
- RDS:
-
Respiratory distress syndrome
- GDM:
-
Gestational diabetes
- ICD-10:
-
International Classification of Diseases-10th Revision
- KNHI:
-
Korea National Health Insurance
- LBW:
-
Low birth weight
- NHSE:
-
National Health Screening Examination
- SGA:
-
Small for gestational age
References
Hughes, M. M., Black, R. E. & Katz, J. 2500-g low birth weight cutoff: History and implications for future research and policy. Matern. Child Health J. 21(2), 283–289 (2017).
Liu, L. et al. Global, regional, and national causes of under-5 mortality in 2000–15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 388(10063), 3027–3035 (2016).
Blencowe, H. et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: A systematic analysis. Lancet Glob. Health. 7(7), e849–e860 (2019).
Tema, T. Prevalence and determinants of low birth weight in Jimma Zone, Southwest Ethiopia. East Afr. Med. J. 83(7), 366–371 (2006).
Suarez-Idueta, L. et al. Vulnerable newborn types: Analysis of population-based registries for 165 million births in 23 countries, 2000–2021. BJOG. 2023.
Shrimpton, R. Preventing low birthweight and reduction of child mortality. Trans. R. Soc. Trop Med. Hyg. 97(1), 39–42 (2003).
Blanc, A. K. & Wardlaw, T. Monitoring low birth weight: An evaluation of international estimates and an updated estimation procedure. Bull. World Health Organ. 83(3), 178–185 (2005).
Upadhyay, R. P. et al. Cognitive and motor outcomes in children born low birth weight: A systematic review and meta-analysis of studies from South Asia. BMC Pediatr. 19(1), 35 (2019).
Leijon, I., Ingemansson, F., Nelson, N., Wadsby, M. & Samuelsson, S. Reading deficits in very low birthweight children are associated with vocabulary and attention issues at the age of seven. Acta Paediatr. 105(1), 60–68 (2016).
Arpi, E. & Ferrari, F. Preterm birth and behaviour problems in infants and preschool-age children: A review of the recent literature. Dev. Med. Child Neurol. 55(9), 788–796 (2013).
Weindrich, D., Jennen-Steinmetz, C., Laucht, M. & Schmidt, M. H. Late sequelae of low birthweight: Mediators of poor school performance at 11 years. Dev. Med. Child. Neurol. 45(7), 463–469 (2003).
Whitaker, A. H. et al. Motor and cognitive outcomes in nondisabled low-birth-weight adolescents: Early determinants. Arch. Pediatr. Adolesc. Med. 160(10), 1040–1046 (2006).
Petrou, S. Economic consequences of preterm birth and low birthweight. BJOG 110(Suppl 20), 17–23 (2003).
Yousafzai, A. K. et al. Effects of responsive stimulation and nutrition interventions on children’s development and growth at age 4 years in a disadvantaged population in Pakistan: A longitudinal follow-up of a cluster-randomised factorial effectiveness trial. Lancet Glob Health. 4(8), e548–e558 (2016).
Sacchi, C. et al. Association of intrauterine growth restriction and small for gestational age status with childhood cognitive outcomes: A systematic review and meta-analysis. JAMA Pediatr. 174(8), 772–781 (2020).
Allotey, J. et al. Cognitive, motor, behavioural and academic performances of children born preterm: A meta-analysis and systematic review involving 64 061 children. BJOG. 125(1), 16–25 (2018).
Wang, W. L. et al. Low birth weight, prematurity, and paternal social status: Impact on the basic competence test in Taiwanese adolescents. J. Pediatr. 153(3), 333–338 (2008).
Paz, I. et al. Term infants with fetal growth restriction are not at increased risk for low intelligence scores at age 17 years. J. Pediatr. 138(1), 87–91 (2001).
O’Keeffe, M. J., O’Callaghan, M., Williams, G. M., Najman, J. M. & Bor, W. Learning, cognitive, and attentional problems in adolescents born small for gestational age. Pediatrics. 112(2), 301–307 (2003).
Cortese, M., Moster, D. & Wilcox, A. J. Term birth weight and neurodevelopmental outcomes. Epidemiology. 32(4), 583–590 (2021).
Emond, A. M., Lira, P. I., Lima, M. C., Grantham-McGregor, S. M. & Ashworth, A. Development and behaviour of low-birthweight term infants at 8 years in northeast Brazil: A longitudinal study. Acta Paediatr. 95(10), 1249–1257 (2006).
Strauss, R. S. Adult functional outcome of those born small for gestational age: Twenty-six year follow-up of the 1970 British Birth Cohort. JAMA. 283(5), 625–632 (2000).
Grantham-McGregor, S. M. Small for gestational age, term babies, in the first six years of life. Eur. J. Clin. Nutr. 52(Suppl 1), S59-64 (1998).
Walker, S. P. et al. Inequality in early childhood: Risk and protective factors for early child development. Lancet. 378(9799), 1325–1338 (2011).
Upadhyay, R. P. et al. Factors determining cognitive, motor and language scores in low birth weight infants from North India. PLoS One. 16(5), e0251387 (2021).
Kratimenos, P. & Penn, A. A. Placental programming of neuropsychiatric disease. Pediatr Res. 86(2), 157–164 (2019).
Kugelman, A. & Colin, A. A. Late preterm infants: Near term but still in a critical developmental time period. Pediatrics. 132(4), 741–751 (2013).
Arima, Y. & Fukuoka, H. Developmental origins of health and disease theory in cardiology. J. Cardiol. 76(1), 14–17 (2020).
Sanz-Cortes, M. et al. Abnormal brain microstructure and metabolism in small-for-gestational-age term fetuses with normal umbilical artery Doppler. Ultrasound Obstet. Gynecol. 36(2), 159–165 (2010).
Jenabi, E., Bashirian, S., Asali, Z. & Seyedi, M. Association between small for gestational age and risk of autism spectrum disorders: A meta-analysis. Clin. Exp. Pediatr. 64(10), 538–542 (2021).
Treluyer, L. et al. Bronchopulmonary dysplasia and risk of developmental delay: An EPIPAGE-2 cohort study. Neonatology. 119(1), 124–128 (2022).
Ballantyne, M., Benzies, K. M., McDonald, S., Magill-Evans, J. & Tough, S. Risk of developmental delay: Comparison of late preterm and full term Canadian infants at age 12 months. Early Hum. Dev. 101, 27–32 (2016).
Choi, W. et al. Association of pre- and perinatal risk factors with tourette syndrome or chronic tic disorders in a korean school-age population. Soa Chongsonyon Chongsin Uihak. 34(1), 37–44 (2023).
Power, C., Jefferis, B. J., Manor, O. & Hertzman, C. The influence of birth weight and socioeconomic position on cognitive development: Does the early home and learning environment modify their effects?. J. Pediatr. 148(1), 54–61 (2006).
Upadhyay, R. P. et al. Early child stimulation, linear growth and neurodevelopment in low birth weight infants. BMC Pediatr. 22(1), 586 (2022).
Lien, N. M., Meyer, K. K. & Winick, M. Early malnutrition and “late” adoption: a study of their effects on the development of Korean orphans adopted into American families. Am. J. Clin. Nutr. 30(10), 1734–1739 (1977).
Lee, C. K. et al. Nationwide validation study of diagnostic algorithms for inflammatory bowel disease in Korean National Health Insurance service database. J. Gastroenterol. Hepatol. 35(5), 760–768 (2020).
Author information
Authors and Affiliations
Contributions
G.J.C. and H.Y.K. had full access to all the data in the study, and they are responsible for the integrity of the data. K.H.A. and S.H. were responsible for statistical analysis and data curation. H.K. and M.O. were responsible for quality assurance and the critical revision of the manuscript for intellectual content. G.J.C. and H.Y.K. was responsible for the concept and design of the study, drafting the manuscript, and the final decision to submit for publication.. All authors were involved in the acquisition, analysis, or interpretation of the data and the critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, H.Y., Cho, G.J., Ahn, K.H. et al. Short-term neonatal and long-term neurodevelopmental outcome of children born term low birth weight. Sci Rep 14, 2274 (2024). https://doi.org/10.1038/s41598-024-52154-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52154-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.