Abstract
This study was conducted to investigate the effects of supplementation of different fat sources in calf starters on growth performance, health, blood fatty acid profiles, and inflammatory markers during the cold season in dairy calves. A total of 48 Holstein calves (24 males and 24 females) were randomly assigned to 1 of 4 starter diets throughout the experiment (d 3 to 65): (1) no supplemented fat (CON), (2) 3% calcium-salts of soybean oil (Ca-SBO), (3) 3% calcium-salts of fish oil (Ca–FO), and (4) 3% mixture of Ca-SBO and Ca–FO (1.5% each, DM basis; MIX). Calves were given free access to starter feed and water and were raised individually in pens from 3 to 65 d of age. Calves fed Ca-SBO consumed a greater proportion of n-6 FA, while calves fed Ca–FO consumed a greater level of n-3 FA compared to the other dietary treatments. Fat supplementation increased the intake of linoleic acid, the major n-6 FA, with the greater intake observed in the Ca-SBO group compared to the other dietary treatments. Calves fed the Ca–FO and MIX diets consumed more long-chain n-3 FA than the other diets. In addition, calves fed Ca-SBO and Ca–FO diets consumed more starter feed and total dry matter than calves fed MIX and CON throughout the experiment (d 3 to 65). Calves fed Ca–FO had higher average daily gain throughout the trial (d 3 to 65) than the other treatment groups. Of all treatment groups, calves fed Ca–FO achieved the highest final body weight and showed the greatest feed efficiency. Random forest analysis revealed that eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and arachidonic acid were the serum levels of FA most affected by the diets. The principal component analysis of blood FA profile, blood parameters, and inflammatory markers showed distinct differences between dietary treatments. Calves fed Ca-SBO had higher plasma concentrations of linoleic acid, while calves fed Ca–FO had higher plasma concentrations of long-chain n-3 polyunsaturated fatty acids (PUFA), such as EPA, docosapentaenoic acid (DPA), and DHA than the other treatment groups. Plasma inflammatory markers were lower in calves fed Ca–FO and higher in calves fed CON than in the other treatment groups. The Ca–FO group had lower levels of inflammatory markers, including serum amyloid A, tumor necrosis factor-alpha, Interferon-γ, haptoglobin, and interleukin-6 compared to the other experimental treatments. Also, the blood malondialdehyde levels, an indicator of oxidative stress, were lower in calves fed Ca–FO compared with calves fed the other treatment diets. In conclusion, the performance of preweaned dairy calves can be improved by adding fat to their starter feed under cold conditions. Overall, the type of fat in milk may affect growth and inflammation of dairy calves before weaning under cold conditions, with n-3 FA (Ca–FO) promoting growth and reducing inflammation more effectively than n-6 FA (Ca-SBO).
Similar content being viewed by others
Introduction
Calves are born with limited body fat stores, and even at 6 weeks old, their body fat percentage remains below 4%1. Calves can respond to low ambient temperatures with thermogenesis, but their body temperature regulation is not as mature as older animals, so cold ambient temperatures are detrimental to their health and survival2,3,4. Calves under cold conditions increase starter feed intake as a biological strategy to provide needed energy5. Previous studies found that calves raised in cold weather consumed more starter feed than calves born in other seasons but had the same weight gain5. These results suggest that this biological strategy cannot provide optimal weight gain for calves raised under cold stress.
Dairy calves were fed supplemental fat to increase their energy intake6. Under mild cold stress conditions, supplementation of milk replacers with fat resulted in greater weight gain at an early age7. Adding fat to the diet of dairy calves has produced conflicting results, with some studies reporting negative effects8, and others reporting positive results9. These discrepancies in calf responses to supplemental fat may be due to several factors, including the type of fat used6,8, the amount of fat added10, and the method of administration11. Additionally, environmental conditions also play a crucial role in the response of animals to added fat4,12,13. Ghasemi et al.12 found that of the different fat sources fed to dairy calves during the cold season (palm fat, soybean oil, tallow, and a mixture of three sources), only soybean oil supplementation improved animal growth performance. Furthermore, previous studies have shown that some individual FA can improve animal immune function9,14.
Although few studies have examined the effects of fat sources in calf starters during cold stress, previous research has not considered the effects of partially protected sources such as calcium salts on calf immune response and inflammatory markers under cold conditions. There is a significant lack of knowledge on whether fat supplementation improves animal growth performance by increasing dietary energy content or regulating inflammatory markers via FA supplementation. It was hypothesized that a starter feed supplemented with fat during the cold season would improve the growth performance of preweaned calves compared to non-supplemented calves. Furthermore, feeding fish oil n-3 FA was hypothesized to result in lower inflammatory markers than calcium salts and soybean oil n-6 FA. Therefore, this study aims to evaluate the effects of calcium salts from soybean oil and fish oil, alone and in combination, on intake, growth performance, health, blood parameters, and inflammatory markers in Holstein dairy calves during the cold season.
Materials and methods
Weather conditions
The experiment was conducted on a commercial dairy farm (approximately 15,000 lactating dairy cattle) in Moghan Agriculture-Industrial and Livestock Co. (Parsabad Moghan, Iran) from December 2018 to February 2019, the typical cold season in the region. Table 1 shows that the average monthly temperature values for December, January, and February at the experimental site were − 2.6 °C, − 3.0 °C, and − 1.7 °C, respectively. The lowest and highest temperatures were recorded in January (− 3.0 °C) and December (11.3 °C), respectively. The highest and lowest monthly humidity were 88.9% and 54.6%, respectively. Animal Care and Use Committee of Urmia University approved all animal experiments (ACUC Protocol #IR2018011) according to the Iranian Council of Animal Care15. The study complies with ARRIVE guidelines for reporting in vivo experiments, and all methods were performed following the relevant guidelines and regulations. Experimental research and field studies on plants (cultivated or wild), including the collection of plant material, must comply with relevant institutional, national, and international guidelines and legislation.
Calves and experimental design
Forty-eight newborn Holstein calves (12 calves/treatment; 6 males and 6 females) with an average age of 3 ± 1 day and a body weight (BW) of 41.8 ± 3.53 kg were assigned in a completely randomized design trial. All calves were assigned to experimental treatments on the same day, and the trial was started on a constant day for all treatments. Calves were raised in individual pens (2.2 m length × 1.1 m width) from 3 to 65 days of age throughout the experiment. Calves received 6 L of colostrum within the first 12 h of life (3 L of colostrum within 2 h of life and 3 L at a second feeding). Colostrum quality was measured using a digital refractometer (Digital dairy model, DD-3, Misco, Cleveland, OH, USA). The colostrum used in the experimental calves exhibited high quality, as evidenced by its Brix index of 25.25 ± 2.54%, total solids content of 27.32 ± 2.53%, and pH of 6.31 ± 0.08. Consequently, the calves had an average serum total protein content of 5.71 ± 0.39 mg/dL on 48 ± 4 h after birth.
Milk was prepared twice daily (at 08:00 and 17:00 h) in steel pails according to the following schedule: 4 L/d from d 3 to 30, 6 L/d from d 31 to 50, 4 L/d from d 51 to 60, and 2 L/d of milk (once daily) during morning feeding from d 61 to 64 of age. Whole milk (38 °C) was pasteurized and routinely analyzed with a milk scanner (MilkoscanTMS50-75610, FOSS Nils Foss Allé 1, DK-3400 Hilleroed, Denmark). The data were used to calculate the DM intake of the calves. The average composition of the milk was 3.3% protein, 3.5% fat, 4.2% lactose, and 13% of total solids.
The experimental treatments were as follows: (1) Starter diet without fat supplementation (CON), (2) 3% Calcium salts of soybean oil (Ca-SBO), (3) 3% Calcium salts of fish oil (Ca–FO), (4) 3% Mixture of SBO and FO (1.5% each, base DM: MIX), Calcium salts of SBO and Calcium salts of FO were provided by Kimiya Danesh Alvand CO (Persia Fat®, Tehran, Iran) as described in Table 2. The ingredients and composition of the experimental diets are described in Table 3. To avoid possible oxidation of FA due to the long storage time, starters were prepared in small batches and at short intervals during the experimental period.
Intake measurement and health indicators recording
Daily measurement of feed intake was done using a calibrated digital scale with a maximum weight capacity of 6 kg ± 0.1 g at 100 mg readability and a minimum piece weight of 5 g (Kern and Sohn, model Kern EW 6000-1 M, Stuttgart, Germany). Body weight was taken using a calibrated digital scale with a maximum capacity of 500 kg ± 100 g (1.5 m length × 1 width, Pand Electronics, Tehran, Iran) every two weeks. The content of feed offered and refused was recorded daily for each calf. Calves were fed textured starters ad libitum to permit at least 10% orts. The ADG (kg of BW/d) was calculated at weighing intervals, and feed efficiency was determined as kg of ADG/total DM intake (DMI; liquid feed DMI + starter feed DMI). Measurements of skeletal growth, including body length, body girth, withers height, hip and pin width, and pin-to-hip width, were done at d 3, 30, and 60 using the method described previously16. Weekly samples of calf starters offered were analyzed for dry matter (No. 934.01), ether extract (No. 954.02), and crude protein (No. 988.05) according to AOAC17. Dietary fiber contents (ADF and NDF) were analyzed following Van Soest et al.18. Chopped alfalfa hay was added to the calf starter diets (10%, DM basis) initiated from d 21. Systematic weekly assessments showed that feed refusals contained an average of 12 ± 3% forage particles.
Rectum and ear skin temperatures were measured and recorded using a digital and infrared thermometer19 (ZOTEK- GM320, Zotek tools, Shenzhen, China). The health scores of the calves were recorded daily according to the calf health chart20. The respiratory clinical score was calculated according to McGuirk and Peek21. Fecal scores were established as (0) normal, (1) semi-formed, pasty, (2) loose, but stays on top of bedding, (3) watery, sifts through bedding. Ear scores: (0) normal, (1) ear flick or head shake, (2) slight unilateral droop, (3) head tilt or bilateral droop. Eye scores: (0) normal, (1) small amount of ocular discharge, (2) moderate amount of bilateral discharge, (3) heavy ocular discharge. Nasal scores: (0) normal serous discharge, (1) small amount of unilateral cloudy discharge, (2) bilateral, cloudy or excessive mucus discharge, (3) copious bilateral mucopurulent discharge. All health incidents, and treatments were recorded for the length of the study.
Fecal samples from healthy calves were collected through rectal palpation into sterile containers on d 58 to 60, before morning milk feeding to count the colony number of major beneficial and pathogenic bacterial species. The pour plate method and MacConkey agar (HiMedia Laboratories Pvt. Limited, India) were used for counting E. coli and Salmonella. Moreover, MRS agar (HiMedia Laboratories Pvt. Limited, India) was used for culturing Lactobacillus species in a veterinary microbiology laboratory (Mabna Lab, Karaj, Iran).
Blood sampling
To collect blood samples from the jugular vein of the calves, 10 mL pre-evacuated tubes were used on day 3, 30, and 60, 3 h after the morning feeding. Two tubes were used for each calf to obtain serum (with no additive in the tube) and plasma samples (heparinized tubes with sodium fluoride and potassium oxalate). The blood samples were transported on ice to the farm adjacent laboratory and allowed to clot at room temperature for 30 min before serum was separated by centrifugation (3000 rpm for 15 min at 4 °C) for serum separation. Heparinized tubes were immediately centrifuged (3000 rpm for 15 min at 4 °C), and plasma samples were segregated. The serum and plasma samples were stored at − 20 °C until subsequent analysis.
Plasma samples were analyzed to determine glucose (intra and inter-assay CV of 1.07 and 1.86, respectively), cholesterol (intra and inter-assay CV of 0.94 and 1.42, respectively), blood urea nitrogen (BUN, intra and inter-assay CV of 2.18 and 2.76, respectively), albumin (intra and inter-assay CV of 0.78 and 1.11, respectively), and total protein (intra and inter-assay CV of 1.45 and 1.88, respectively) using commercially available diagnostic kits (Biorex-Fars, Shiraz, Iran) and a calibrated autoanalyzer (BT-1500, Biotecnica Instruments, Rome, Italy). The concentrations of inflammatory markers were measured in the serum of calves using ELISA kits and an ELISA reader (DANA 3200, Garny, Iran) as follows:
-
Bovine serum tumor necrosis factor-alpha (TNF-α, Bioassay, Shanghai, China, Cat No: E0019Bo, intra and inter-assay CV of 1.53 and 4.18, respectively)
-
Bovine serum amyloid A (SAA, Bioassay, Shanghai, China, Cat No: E0023Bo, intra and inter-assay CV of 2.18 and 6.32, respectively)
-
Interferon-gamma (IFN-γ, Karmania Pars Gene, Kerman, Iran, intra and inter-assay CV of 2.18 and 2.76, respectively)
-
Interleukin-6 (IL-6, Karmania Pars Gene, Kerman, Iran, intra and inter-assay CV of 1.43 and 3.24, respectively)
An immunoturbidimetric assay22 as performed to determine serum haptoglobin (Hp) concentration (anti-bovine immunoturbidimetric assay; Biorex-Fars, Shiraz, Iran, intra and inter-assay CV of 4.6 and 4.81, respectively) using an automated biochemical analyzer (BT-1500, Biotecnica Instruments, Rome, Italy). Malondialdehyde (MDA, Nalondi™-Lipid Peroxidation Assay Kit-MDA; Navand-salamat, Urmia, Iran) was also measured in serum samples using a plate reader (DANA 3200, Garny, Iran) at 550 nm.
Fatty acid profile of plasma samples was determined only from the samples obtained from 60-day-old calves. All the chemical solvents and reagents utilized in lipid extraction and preparation of the fatty acid methyl esters (FAME) were of analytical grade, and solvents were redistilled before use. As described in Folch et al.23 to avoid fatty acid oxidation, lipid extraction was carried out three times with chloroform/methanol (C/M, 2/1, v/v) to a final volume of 100 mL administered under the argon gas blanket. The flasks, after each extraction step, were centrifuged (1800 g for 10 min), and the organic fraction was separated and injected into a 100 mL volumetric flask. Afterward, they were treated with anhydrous Na-sulfate to be dry and then vaporized using a rotary evaporator (Büchi, Switzerland) at 40 °C under a vacuum. Using mild methanolysis/methylation via methanolic hydrochloric acid (HCl/MeOH), FAME were prepared by a method explained by Ichihara and Fukubayashi24. Hexane was utilized as a solvent to extract, and gas chromatography analysis was conducted after drying with anhydrous Na-sulfate. Nonadecanoic acid was utilized as an internal standard.
For FA analysis, Agilent 6890 gas chromatograph (Agilent Technologies, Santa Clara, California, United States) equipped with an autoinjector (Agilent 7683 series, Santa Clara, California, United States) and FID detector was used. Samples (1 µL) were injected in split mode, 50:1, into a RESTEK column for FAME (Rtx®-2330, 105 m × 250 µm × 0·2 µm; catalog No. 10729; serial No. 1525353, Restek Corporation, 110 Benner Circle, Bellefonte, PA16823). The detector and injector temperatures were set at 250 °C. The carrier gas was N2 at a flow of 1 mL/min. Based on the method described by Lee et al.25, the oven temperature was set at the gradient temperature to rise with some modifications. It was 70 °C for 1 min, and then was increased from 5 °C/min to 100 °C and was kept for 2 min. Then, the column temperature was increased from 10 °C/min to 175 °C and was maintained for 35 min. The temperature was increased from 4 °C/min to 225 °C and was kept for 35 min. Based on a FAME standard mix (GLC 463, Nu-Chek Prep Inc., Elysian, MN; reference mixture 47 885, Supelco Inc., Bellefonte PAGLC 463 reference mixture, http://www.nu-chekprep.com/10 11 catalog.pdf), individual peaks were identified.
Statistical analysis
A standard deviation of 100 g ADG was assumed, with the minimum meaningful difference in ADG set at 65–75 g/d, based on the values published previously26,27. A power test analysis was run (α = 0.05 and power (1–β) = 0.80), and the expected sample size of 12 calves/treatment was achieved for growth performance, the most reliable parameter for power test determination28,29. Repeated measures variables (starter feed intake, DMI, FA intake, ADG, feed efficiency, skeletal growth parameters) within the same calf were analyzed with fixed effects of treatment, time (d), the interaction between treatment and time, and sex of calf, and random effect of calf nested within treatment.
To determine the most appropriate variance–covariance structure for our analysis, three variance–covariance structures (autoregressive type 1, compound symmetry, and Toeplitz) were tested, and an autoregressive type 1 covariance structure was selected as the best fit based on the Bayesian information criterion. All residuals were tested for normality using the Shapiro–Wilk statistic and the UNIVARIATE procedure of SAS (version 9.4, SAS Institute Inc., Cary, NC), and for homogeneity of variance using Levene's test, and visually assessed using quantile–quantile plots. Data (starter feed intake, DMI, ADG, BW, skeletal growth, and fecal scores) that did not meet the assumptions of normality of the residuals had to be log-transformed (base 10). After log transformation, the distribution of the data was retested, and the data were normally distributed.
The data for plasma metabolites and fecal microbial counts were analyzed using the above mixed-effects model without the effect of time. The initial values of body measures (BW and skeletal growth) were used as covariates for the corresponding variables. Data were reported as least squares mean, and the Tukey–Kramer adjustment was applied to account for multiple comparisons. The SLICE statement of the MIXED procedure (PROC MIXED) of SAS was used to conduct partitioned analyses of the LSM for interactions when required. The data for health and fecal score were analyzed by logistic regression using a binomial distribution in the GLIMMIX procedure in SAS. The significance threshold was set at P ≤ 0.05; trends were declared at 0.05 < P ≤ 0.10.
Multivariate analyses
Multivariate statistical analyses of blood parameters of calves fed different diets were performed using the web-based metabolomics data processing tool MetaboAnalyst 5.0 (Pang et al.,30; http://www.metaboanalyst.ca for detailed methodology). Data were transformed using the generalized square root transformation and then scaled by Pareto (mean divided by the square root of the standard deviation of each variable) to correct for heteroscedasticity, reduce skewness, and mask effects. Principal component analysis (PCA) was applied to obtain an overview of blood parameters including albumin, cholesterol, glucose, haptoglobin, IFN-γ, IL-6, MDA, SAA, TNF-α, total protein, and urea according to treatment on d 30 and d 60. Identification of significant blood metabolites affected by the dietary treatments was performed using random forest classification. In this process, 500 trees were used and each node had seven predictors using MetaboAnalyst 5.030.
Results
Intake and growth performance
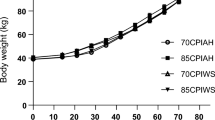
Table 4 shows the results for starter feed intake, DMI, and FA intake from starter diets. Starter feed intake interacted with fat source and age. At week 2, calves fed Ca-SBO had greater starter feed intake than calves fed other feeds. At weeks 5 through 9, calves fed Ca–FO had greater starter feed than calves fed CON and MIX (Fig. 1A). Calves fed Ca-SBO and Ca–FO had greater starter feed intake and total DMI than calves fed MIX and CON throughout the experiment. Fat source and time interacted to affect the intake of FA from the starter diet at weeks 5 to 9 (Table 4). Calves fed Ca-SBO had the greater intake of C18:2 and C18:1, followed by the MIX and Ca–FO groups. Intake of C18:3 was greater in the Ca-SBO group, followed by the Ca–FO and MIX groups. The intake of C18:0 was greater in the Ca–FO group, followed by the MIX and Ca-SBO groups. Intake of EPA and DHA was greater in calves fed Ca–FO and MIX than the other diets. Fat source and age affected ADG (Table 5). Calves fed Ca–FO had higher ADG than the other treatments on d 28, 56, and 64. Calves fed Ca–FO had a significant increase in BW on d 28, 56, and 64 compared to the other treatments (Fig. 1B). The Ca–FO group showed greater feed efficiency throughout the experimental period (d 3—65), followed by the MIX, Ca-SBO and CON groups.
Effect of different fat sources on starter feed intake (A) and body weight (B) in dairy calves fed different diets. The four treatments included (1) a control group with no supplemented fat (CON), (2) a group fed 3% calcium salts of soybean oil (Ca-SBO), (3) a group fed 3% calcium salts of fish oil (Ca–FO), and (4) a group fed a mixture of 1.5% Ca-SBO and 1.5% Ca–FO on a dry matter basis (MIX). Symbols indicate a difference (*P = 0.01; **P < 0.01) between the groups at a given time. Data are presented as means ± SEM. The significance threshold was set at P ≤ 0.05; trends were declared at 0.05 < P ≤ 0.10.
None of the structural growth indices, including withers height, body length, body girth, pin width, and hip width, were affected by fat supplementation during the experimental period.
Blood FA profiles
Considering the results of the plasma FA profile of the calves at d 60 revealed that the concentration of linoleic acid was greater in calves fed the Ca-SBO diet compared with the other treatments (Table 6). Blood concentrations for long-chain n-3 polyunsaturated FA (PUFA) including EPA and DHA were greater in Ca–FO than in the other treatments, but dietary supplementation with fat did not affect plasma concentrations of linolenic acid (C18:3). Based on the serum FA composition, PCA analyses (Fig. 2A) showed a clear distinction between the dietary treatments on d 60. The first principal component (PC) accounted for 46.3% of the variance, whereas the second and third PCs contributed 15.4% and 12.4%, respectively. In addition, random forest analyses revealed that EPA, DHA, and arachidonic acid were the serum FA most affected by treatment diets, with the greater levels observed in calves fed Ca–FO than in the other treatments (Fig. 2B).
Principal component analysis (PCA) plot (A) and random forest (B) of serum fatty acid composition in dairy calves fed different diets. The four treatments included (1) a control group with no supplemented fat (CON), (2) a group fed 3% calcium salts of soybean oil (Ca-SBO), (3) a group fed 3% calcium salts of fish oil (Ca–FO), and (4) a group fed a mixture of 1.5% Ca-SBO and 1.5% Ca–FO on a dry matter basis (MIX).
Blood metabolites, and inflammatory markers
As shown in Table 7, calves fed the CON diet had significantly lower blood glucose concentrations than calves fed the other diets. Blood cholesterol concentrations were significantly greater in calves fed the Ca–FO diet than in calves fed the other diets. Fat supplementation significantly increased blood cholesterol concentrations compared to the CON diet. Calves fed the Ca-SBO diet had significantly lower total protein concentrations than calves fed the other diets.
Inflammatory markers such as TNF-α, INF-γ, Hp, SAA, and IL-6 had the lower concentrations in the blood of calves fed Ca–FO compared with the other experimental treatments. In contrast, calves fed CON had the greater concentrations of TNF-α, Hp, and SAA in blood during the experimental period compared to calves fed the other treatment diets. Blood MDA concentrations (an indicator of oxidative stress) were lower in calves fed Ca–FO compared to calves fed the other treatment diets. The PCA analysis of blood metabolites and inflammatory markers showed that there were distinct differences between dietary treatments at d 30 and 60 of the study (Fig. 3A,B). At d 30 (Fig. 3A), the first PC explained 44% of the variance, and the second and third PC explained 11.9% and 10.3%, respectively (PC2 and PC3 = 22.2%). According to Fig. 3B, PC1 explained 48.4% of the variance, whereas PC2 and PC3 contributed 10.2% and 9.3%, respectively (PC2 and PC3 = 19.5%). Random forest analysis was used to identify changes in blood inflammatory markers in response to dietary treatment at d 30 (Fig. 4A) and d 60 (Fig. 4B). Results showed that SAA, TNF-α, and INF-γ were the most affected serum FA on d 30, whereas SAA, total protein, and TNF-α were the most affected on d 60, with calves fed Ca–FO having the lowest levels of inflammatory markers (Fig. 4A,B).
Principal component analysis (PCA) plot of blood metabolites, and inflammatory markers on d 30 (A) and d 60 (B) in Holstein calves fed different diets. The four treatments included (1) a control group with no supplemented fat (CON), (2) a group fed 3% calcium salts of soybean oil (Ca-SBO), (3) a group fed 3% calcium salts of fish oil (Ca–FO), and (4) a group fed a mixture of 1.5% Ca-SBO and 1.5% Ca–FO on a dry matter basis (MIX).
Random forest plot of blood metabolites, and inflammatory markers on d 30 (A) and d 60 (B) in Holstein calves fed different diets. The four treatments included (1) a control group with no supplemented fat (CON), (2) a group fed 3% calcium salts of soybean oil (Ca-SBO), (3) a group fed 3% calcium salts of fish oil (Ca–FO), and (4) a group fed a mixture of 1.5% Ca-SBO and 1.5% Ca–FO on a dry matter basis (MIX).
Health indicators and fecal microbial counts
Table 8 summarizes the results of health indicators and fecal microbial counts. Supplementation with Ca–FO resulted in lower nasal discharge indices compared with the CON diet. The addition of Ca–FO and Ca-SBO to the starter diet resulted in the lower ear and eye scores compared with the CON diet, but the MIX diet did not improve health indices. In addition, supplementation of the starter feed with Ca–FO had a lower rectal temperature, ear skin temperature, and respiratory rate compared with calves fed the CON feed. It was also found that calves fed the CON feed had higher E. coli counts than calves fed the supplemental fat feed.
Discussion
The lower critical temperature for rearing calves is 15 °C31. In the current study, the ambient temperature was below this value, indicating persistent cold stress for the calves. Cold stress can occur in the first month of calf rearing below 10 °C, and temperatures below -2 °C cause cold stress until weaning. Calves in cold climates have increased metabolic rates and lower critical temperatures32,33, which has negative effects due to immature cold protection mechanisms and high energy requirements34,35. This stress results in lower calf performance and economic losses in the calf industry36,37. The nutritional needs of calves raised below their thermoneutral zone are difficult to meet because much of the feed energy is used for thermoregulation, leaving less energy for growth promotion37,38.
Intake, growth performance, and blood FA profile
Young calves have only about 3% body fat reserves and therefore dietary energy supplementation is necessary to avoid the negative consequences of energy deficits caused by cold stress3. Supplemental fat may be beneficial for calves suffering from cold stress because it conserves glucose and AA for thermoregulation, which is critical when calves are exposed to temperatures below the thermal neutral zone7. In addition, extra fat can conserve energy that can be used for functions other than growth, including immune function39. However, there are limited data on the effects of supplemental fat on pre-weaned calves under cold stress. In the current study, calves given 3% Ca-SBO, Ca–FO, or a mix had higher starter feed intake compared with the CON group, indicating the potential advantage of high-fat diets in cold weather. Our data indicate that young calves increase their energy intake during cold stress, and feed intake is not limited by metabolic factors as fat supplements (3% of the stater diet) are fed. Holt (2014) reported that calves raised in winter months consumed more calf starter than did those born in other months. In dairy cows, fat supplements differ markedly in their effects on DMI.
Researchers have previously reported that chronic cold stress can activate various inflammatory mechanisms and eventually increase circulating inflammatory cytokines40,41,42. Although this is the first study to report the effects of inflammation on preweaning feed intake of dairy cows, Kuhla43 and Gouvêa et al.,44 have found in separate reviews that pro-inflammatory cytokines, hypothalamic inflammation, and stress-induced inflammation negatively affect feed intake. As shown in Table 7, supplementation with Ca–FO results in lower inflammatory indices compared with control and Ca-SBO or MIX treatments, which may be an effective mechanism to explain the higher dry matter intake (DMI) of calves supplemented with Ca–FO.
As expected, feeding Ca-SBO to pre-weaned calves resulted in increased intake of C18 (linoleic and linolenic acids) PUFA, whereas Ca–FO resulted in increased intake of long-chain n-3 PUFA (EPA, and DHA) compared with CON. Calf starter diets typically contain large amounts of corn grain and soybean meal, which have a high proportion of linoleic acid in their fat content as n-6 FA, resulting in a high n-6: n-3 ratio (approximately 15:1,8). However, feeding soybean oil did not change this ratio or have a positive effect on starter feed intake and the growth performance of dairy calves45. This negative effect was observed in dairy calves when starter feeds high in n-6 FA was ingested, possibly due to the pro-inflammatory effect of n-6 FA46. Researchers have reported that supplementation with various n-3 FA sources, such as milk replacers with fish oil and linseed oil47, can improve the intake and growth performance of calves before weaning. The results of our study suggest that cold weather increases animal appetite, resulting in a higher intake of starter feeds when supplemented with fat compared with low-fat feeds. In addition, our results suggest that n-3 FA may have a more favorable effect on the starter feed intake compared to n-6 FA during the cold season.
The current study showed that feeding Ca–FO resulted in higher ADG, feed efficiency, and final weight because the starter feed provided more energy and other nutrients for weight gain. Our results are consistent with previous studies reporting that feeding n-3 FA in the form of Ca salts from flaxseed oil in the calf starter before weaning improved weight gain and feed efficiency compared to control diets48,49. In addition, studies by Hill et al.6 and Kadkhoday et al.50 showed that calves fed linseed oil had higher feed efficiency compared with calves fed the control diet. Mohtashami et al.13 also found that feed efficiency was improved in calves fed Ca salts of fish oil compared to pre-weaned calves fed Ca salts of soybean oil under heat stress. Although little information is available on calves, at least some of the improved average daily gain (ADG) and feed efficiency observed in calves supplemented with Ca–FO may be related to their inflammatory status and the effects of systemic inflammation on growth hormone and the growth hormone-insulin-like growth factor-1 (IGF-1) axis51,52.
Dietary PUFA can alter several physiological processes, including plasma lipid levels, immune function, insulin action, and cell regulation53. As previous studies have shown54,55,56, neonates rapidly increase their ability to oxidize FA after birth. As a result, increased PUFA intake in the preweaning period may increase the ability to oxidize FA, promote gene expression for oxidation, and decrease esterification, leading to changes in body composition. A study by Melendez et al.57 compared the effect of n-3 FA in milk replacers with that of n-6 and n-9 FA in canola oil on serum FA profiles in preweaning calves. They found that calves fed fish oil had higher n-3 FA concentrations than calves fed canola oil, but the sum of n-6 and n-9 FA did not differ in serum between treatments. According to our results, the observed differences in plasma FA composition could be seen as reflecting differences in diet., as shown by the higher intake of C16:0, C18:0, C18:1, and C18:3 FA in calves fed Ca–FO and Ca-SBO compared with those fed CON, with the lowest intake of all FA except C18:2. In addition, the Ca-SBO diet increased in blood n-6 FA, while the Ca–FO and MIX diets increased DPA, EPA, and DHA levels, as expected.
As part of the analysis of blood FA data, both PCA plots and random forest analysis were used. According to the PCA plots, there was a clear separation between the different treatment groups, with significant differences in calves fed the Ca–FO diet and the MIX diet. In addition, random forest analysis showed that long-chain n-3 PUFA, including DPA, DHA, and 20:4 (n-6), were most affected by the diets. Calves fed the Ca–FO diet had the greater concentrations of PUFA than calves fed the other diets, which were strongly related to the fat content of the starter diet. These results suggest that the inclusion of n-3 PUFA in the diets of calves before weaning, particularly in the form of fish oil, can significantly alter the FA profile of their blood and increase the concentration of important long-chain n-3 PUFA.
Blood parameters and inflammatory markers
Calves fed the CON diet had lower blood glucose concentrations than calves fed the other diets. The Ca–FO with supplementation of omega-3 FA had a better effect on increasing plasma glucose levels, which can be attributed to the increased intake of starter feed. Starter feed intake plays the main role in regulating blood glucose concentration in dairy calves58. Ghasemi et al.12 reported that calves fed SBO had higher glucose concentrations than calves without supplementation. It has been suggested that glucose can be saved by fat supplementation and used for other purposes12. In addition, previous studies have shown that supplementation with long-chain n-3 FA increases hepatic glycerol gluconeogenesis and thus positively stimulates hepatic glucose production in humans and laboratory animals59,60, which needs further investigation in ruminants. Blood cholesterol concentration was increased in calves fed fat supplemented starter diets compared with CON, which is consistent with previous studies on calves fed supplemental fat12,61. Thus, our results suggest that higher blood cholesterol concentrations in calves raised during the cold season may have a preventive effect against diarrhea. Further studies are needed to elucidate the mechanisms involved. The lowest TP was observed in calves fed Ca-SBO in the current study. This is likely due to the higher requirement for lipoprotein synthesis to transport absorbed lipids in the blood observed in calves fed high-fat starter diets62. It is worth noting that previous studies on dairy calves have recommended feeding high protein to improve nitrogen intake in calves fed soybean oil45.
The study found that calves fed Ca–FO had lower concentrations of inflammatory markers (TNF-α, INF-γ, Hp, SAA, and IL-6) and MDA (an indicator of oxidative stress) compared to other experimental treatments. This indicates that the inclusion of fish oil, with its anti-inflammatory effects and n-3 fatty acids, benefits calves raised in cold preweaning conditions. On the other hand, the CON diet resulted in higher concentrations of TNF-α, Hp, and SAA, suggesting the pro-inflammatory properties of this particular diet in the study. The relevant literature supports these findings by highlighting the numerous metabolic activities of the long-chain FA present in fish oil that contribute to their anti-inflammatory effects63. The mechanisms involved in the regulation of inflammatory effects are not fully understood, but evidence suggests that long-chain FA activates cellular communication through the synthesis of prostaglandins, TNF-α, and IFN-γ, and influences other factors such as nitric oxide64. The lowest MDA level as an indicator of oxidative stress was found in the Ca–FO diet compared with other diets, suggesting that PUFA in fish oil calcium salts is not susceptible to oxidation during the cold season. While the specific mechanisms behind these effects need further investigation, the results provide substantial evidence for the superiority of Ca–FO in terms of its beneficial effects on inflammation and oxidative stress in such cold conditions. Breakdown of the dietary lipids to free fatty acids occurs rapidly and, in fact, more rapidly than the biohydrogenation process. Thus, large amounts of unsaturated oils can “overwhelm” the biohydrogenation process and result in undesirable effects on the rumen microbial population. During the biohydrogenation process, intermediate compounds with trans-double bonds are produced. One of these is conjugated linoleic acid (CLA), which has received much attention from the medical community because of its potent anti-cancer effects and other health benefits.
Health indicators and fecal microbial counts
Calves receiving Ca–FO had more favorable fecal scores and fewer days with diarrhea than other experimental treatments, partly due to the effects of n-3 FA on animal health. When comparing FA in the starter feeds, results showed that n-6 FA was less effective in improving fecal scores than n-3 FA. Previous studies indicated that feeding soybean oil, which is rich in n-6 FA, to young calves may increase fecal fluidity and the animals showed more dietary diarrhea during the experimental period11. In contrast, Kadkhoday et al.50 indicated that milk-fed calves fed n-3 FA-rich flaxseed oil in the starter diet had better fecal scores during the study. In the study conducted by Garcia et al.49, diarrhea severity showed a linear tendency to decrease with increasing essential FA intake.
Lower rectal and ear skin temperatures in calves fed Ca–FO in starter feed indicated better health indices when calves received this FA supplement. Previous studies showed lower rectal temperature when calves were fed linseed oil, which is rich in n-3 FA65. This may be mainly related to the pro-inflammatory effect of n-6 FA (linoleic acid) and the anti-inflammatory effect of n-3 FA6,9,49. In this study, the number of E. coli was higher in calves fed CON forage. This suggests that feeding high-fat diets in the cold season makes calves less susceptible to pathogens, regardless of the FA source.
These results highlight the importance of selecting appropriate fat sources and providing optimal levels of essential FA in calf diets to promote health and growth. Further research is needed to elucidate the mechanisms underlying these effects and to optimize nutritional strategies for rearing dairy calves.
Conclusions
The study showed that the addition of fat to starter feed can improve the performance of dairy calves before weaning under cold conditions. However, the type of fat used affected growth performance and inflammatory status. Specifically, supplementation with Ca–FO resulted in significant improvements in ADG, BW, feed efficiency, and fecal scores. In contrast, CON had higher levels of IL-6, haptoglobin, TNF-α, and SAA, along with lower blood glucose and cholesterol levels. These results suggest that supplementation with n-3 FA (Ca–FO) provides greater benefits in promoting growth and reducing inflammation in dairy calves exposed to cold conditions compared to n-6 FA (Ca-SBO).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Diesel, D. A., Lebel, J. L. & Tucker, A. Pulmonary particle deposition and airway mucociliary clearance in cold-exposed calves. Am. J. Vet. Res. 52, 1665–1671 (1991).
Olson, D. P., Papasian, C. J. & Ritter, R. C. The effects of cold stress on neonatal calves. I. Clinical condition and pathological lesions. Can. J. Comp. Med. 44, 11–18 (1980).
Davis, C. D. & Drackley, J. K. The Development, Nutrition, and Management of the Young Calf (Iowa State University Press, 1998).
Moons, C. P. H., Sonck, B. & Tuyttens, F. A. M. Importance of outdoor shelter for cattle in temperate climates. Livest. Sci. 159, 87–101. https://doi.org/10.1016/j.livsci.2013.11.003 (2014).
Holt, S. D. Ambient temperature, calf intakes, and weight gains on preweaned dairy calves. MS thesis. Animal, Dairy, and Veterinary Sciences, Utah State University, Logan. Accessed Jun. 10. https://doi.org/10.26076/8c38-c14e (2014).
Hill, T. M., Bateman, H. G., Aldrich, J. M. & Schlotterbeck, R. L. Effect of various fatty acids on dairy calf performance. Prof. Anim. Sci. 27, 167–175. https://doi.org/10.15232/S1080-7446(15)30470-8 (2011).
Litherland, N. B., Da Silva, D. N., LaBerge, R. J., Schefers, J. & Kertz, A. Supplemental fat for dairy calves during mild cold stress. J. Dairy Sci. 97, 2980–2989. https://doi.org/10.3168/jds.2013-6942 (2014).
Hill, T. M., Bateman, H. G., Aldrich, J. M., Quigley, J. & Schlotterbeck, R. Inclusion of tallow and soybean oil to calf starters fed to dairy calves from birth to four months of age on calf performance and digestion. J. Dairy Sci. 98, 4882–4888. https://doi.org/10.3168/jds.2015-9376 (2015).
Garcia, M. et al. Effect of supplementing essential fatty acids to pregnant nonlactating Holstein cows and their preweaned calves on calf performance, immune response, and health. J. Dairy Sci. 97, 5045–5064. https://doi.org/10.3168/jds.2013-7473 (2014).
Quigley, J. D. et al. Effects of fatty acids and calf starter form on intake, growth, digestion, and selected blood metabolites in male calves from 0 to 4 months of age. J. Dairy Sci. 102, 8074–8091. https://doi.org/10.3168/jds.2019-16688 (2019).
Ghorbani, H., Kazemi-Bonchenari, M., HosseinYazdi, M. & Mahjoubi, E. Effects of various fat delivery methods in starter diet on growth performance, nutrients digestibility and blood metabolites of Holstein dairy calves. Anim. Feed. Sci. Tech. 262, 114429. https://doi.org/10.1016/j.anifeedsci.2020.114429 (2020).
Ghasemi, E., Azad-Shahraki, M. & Khorvash, M. Effect of different fat supplements on performance of dairy calves during cold season. J. Dairy Sci. 100, 1–10. https://doi.org/10.3168/jds.2016-11827 (2017).
Mohtashami, B. et al. The effect of supplemental bioactive fatty acids on growth performance and immune function of milk-fed Holstein dairy calves during heat stress. Brit. J. Nutr. 127, 188–201. https://doi.org/10.1017/S0007114521000908 (2022).
Karcher, E. L. et al. Comparison of supplementation of n-3 fatty acids from fish and flax oil on cytokine gene expression and growth of milk-fed Holstein calves. J. Dairy Sci. 97, 2329–2337. https://doi.org/10.3168/jds.2013-7160 (2014).
Iranian Council of Animal Care. Guide to the Care and Use of Experimental Animals Vol. 1 (Isfahan University of Technology, 1995).
Khan, M. A. et al. Structural growth, rumen development, and metabolic and immune responses of Holstein male calves fed milk through step-down and conventional methods. J. Dairy Sci. 90, 3376–3387. https://doi.org/10.3168/jds.2007-0104 (2007).
AOAC. Official Methods of Analysis of AOAC International. Volume 1. 17th Edition. Ed. Horwitz, W., AOAC International® (2000).
Van Soest, P. V., Robertson, J. & Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2 (1991).
Fu-Kang, W., Ju-Yin, Sh., Pin-Hsun, J., Ya-Chi, S. & Yu-Chieh, W. Non-invasive cattle body temperature measurement using infrared thermography and auxiliary sensors. Sensors. 21, 1–16. https://doi.org/10.3390/s21072425 (2021).
Swartz, T. H., Findlay, A. N. & Petersson-Wolfe, C. S. Short communication: Automated detection of behavioral changes from respiratory disease in pre-weaned calves. J. Dairy Sci. 100, 9273–9278. https://doi.org/10.3168/jds.2016-12280 (2017).
McGuirk, S. & Peek, S. Timely diagnosis of dairy calf respiratory disease using a standardized scoring system. Anim. Health Res. Rev. 15, 145–147. https://doi.org/10.1017/S1466252314000267 (2014).
Brady, N., O’Reilly, E. L., McComb, C., Macrae, A. I. & Eckersall, P. D. An immunoturbidimetric assay for bovine haptoglobin. Comput. Clin. Path. 28(1), 21–27. https://doi.org/10.1007/s00580-018-2863-6 (2019).
Folch, J., Lees, M. & Sloane-Stanley, G. H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509. https://doi.org/10.1016/s0021-9258(18)64849-5 (1957).
Ichihara, K. & Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid. Res. 51, 635–640. https://doi.org/10.1194/jlr.D001065 (2010).
Lee, M. R. F., Tweed, J. K. S., Moloney, A. P. & Scollan, N. D. The effects of fish oil supplementation on rumen metabolism and the biohydrogenation of unsaturated fatty acids in beef steers given diets containing sunflower oil. Anim. Sci. 80, 361–367. https://doi.org/10.1079/ASC41920361 (2005).
DeVries, T. J. & von Keyserlingk, M. A. Competition for feed affects the feeding behavior of growing dairy heifers. J. Dairy Sci. 92, 3922–3929. https://doi.org/10.3168/jds.2008-1934 (2009).
Miller-Cushon, E. K. & DeVries, T. J. Effect of early feed type exposure on diet-selection behavior of dairy calves. J. Dairy Sci. 94, 342–350. https://doi.org/10.3168/jds.2010-3382 (2011).
Morris, T. R. Experimental Design and Analysis in Animal Sciences (CABI Publishing, 1999).
Hintze J. PASS 2008. NCSS, LLC. Kaysville, UT. Available online at www.ncss.com; accessed August 27, (2015).
Pang, Z. et al. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 49(W1), 388–396. https://doi.org/10.1093/nar/gkab382 (2021).
NRC. Nutrient Requirements of Dairy Cattle, 7th rev. (National Academy Press, 2001).
Young, B. A. Ruminant cold stress: Effect on production. J. Anim. Sci. 57, 1601–1607 (1983).
Robinson, J. B., Ames, D. R. & Milliken, G. A. Heat production of cattle acclimated to cold, thermoneutrality and heat when exposed to thermoneutrality and heat stress. J. Anim. Sci. 62, 1434–1440. https://doi.org/10.2527/jas1986.6251434x (1986).
Gonzalez-Jimenez, E. & Blaxter, K. L. The metabolism and thermal regulation of calves in the first month of life. Brit. J. Nutr. 16, 199–212. https://doi.org/10.1079/bjn19620021 (1962).
Scibilia, L. S., Muller, L. D., Kensinger, R. S., Sweeney, T. F. & Shellenberger, P. R. Effect of environmental temperature and dietary fat on growth and physiological responses of newborn calves. J. Dairy Sci. 70, 1426–1433. https://doi.org/10.3168/jds.S00220302(87)80165-0 (1987).
Nonnecke, B. J. et al. Effects of chronic environmental cold on growth, health, and select metabolic and immunologic responses of preruminant calves. J. Dairy Sci. 92, 6134–6143. https://doi.org/10.3168/jds.2009-2517 (2009).
Roland, L., Drillich, M., Klein-Jöbstl, D. & Iwersen, M. Invited review: Influence of climatic conditions on the development, performance, and health of calves. J. Dairy Sci. 99, 2438–2452. https://doi.org/10.3168/jds.2015-9901 (2016).
Drackley, J. K. Calf nutrition from birth to breeding. Vet. Clin. North Am. Food Anim. Pract. 24, 55–86. https://doi.org/10.1016/j.cvfa.2008.01.001 (2008).
Nonnecke, B. J., Foote, M. R., Smith, J. M., Pesch, B. A. & Van Amburgh, M. E. Composition and functional capacity of blood mononuclear leukocyte populations from neonatal calves on standard and intensified milk replacer diets. J. Dairy Sci. 86, 3592–3604. https://doi.org/10.3168/jds.S0022-0302(03)73965-4 (2003).
Kaushik, S. & Kaur, J. Effect of chronic cold stress on intestinal epithelial cell proliferation and inflammation in rats. Stress. 8, 191–197. https://doi.org/10.1080/10253890500245953 (2005).
Teng, T. et al. Activation of inflammatory networks in the lungs caused by chronic cold stress is moderately attenuated by glucose supplementation. Int. J. Mol. Sci. 23, 10697. https://doi.org/10.3390/ijms231810697 (2022).
Lv, H., He, Y., Wu, J., Zhen, L. & Zheng, Y. Chronic cold stress-induced myocardial injury: Effects on oxidative stress, inflammation and pyroptosis. J Vet Sci. 24, e2. https://doi.org/10.4142/jvs.22185 (2023).
Kuhla, B. Review: Pro-inflammatory cytokines and hypothalamic inflammation: implications for insufficient feed intake of transition dairy cows. Animal. 14(S1), s65–s77. https://doi.org/10.1017/S1751731119003124 (2020).
Gouvêa, V. N., Cooke, R. F. & Marques, R. S. Impacts of stress-induced inflammation on feed intake of beef cattle. Front. Anim. Sci. 3, 962748. https://doi.org/10.3389/fanim.2022.962748.(2022) (2022).
Yousefinejad, S. et al. Soybean oil supplementation and starter protein content: Effects on growth performance, digestibility, ruminal fermentation, and urinary purine derivatives of Holstein dairy calves. J. Dairy Sci. 104, 1630–1644. https://doi.org/10.3168/jds.2020-18823 (2021).
Tsai, C. Y., Rezamand, P., Loucks, W. I., Scholte, C. M. & Doumit, M. E. The effect of dietary fat on fatty acid composition, gene expression and vitamin status in pre-ruminant calves. Anim. Feed. Sci. Tech. 229, 32–42. https://doi.org/10.1016/j.anifeedsci.2017.05.004 (2017).
Karcher, E. et al. Comparison of supplementation of n-3 fatty acids from fish and flax oil on cytokine gene expression and growth of milk-fed Holstein calves. J. Dairy Sci. 97(4), 2329–2337. https://doi.org/10.3168/jds.2013-7160 (2014).
Hill, T. M., Bateman, H. G., Aldrich, J. M. & Schlotterbeck, R. L. Effects of changing the essential and functional fatty acid intake of dairy calves. J. Dairy Sci. 92, 670–676. https://doi.org/10.3168/jds.2008-1368 (2009).
Garcia, M. et al. Increasing intake of essential fatty acids from milk replacer benefits performance, immune responses, and health of preweaned Holstein calves. J. Dairy Sci. 98, 458–477. https://doi.org/10.3168/jds.2014-8384 (2015).
Kadkhoday, A., Riasi, A., Alikhani, M., Dehghan-Banadaky, M. & Kowsar, R. Effects of fat sources and dietary C18:2 to C18:3 fatty acids ratio on growth performance, ruminal fermentation and some blood components of Holstein calves. Livest. Sci. 204, 71–77. https://doi.org/10.1016/j.livsci.2017.08.012 (2017).
Cirillo, F., Lazzeroni, P., Sartori, C. & Street, M. E. Inflammatory diseases and growth: Effects on the GH-IGF axis and on growth plate. Int. J. Mol. Sci. 18, 1878. https://doi.org/10.3390/ijms18091878 (2017).
DeBoer, M. D. et al. Systemic inflammation, growth factors, and linear growth in the setting of infection and malnutrition. Nutrition. 33, 248–253. https://doi.org/10.1016/j.nut.2016.06.013 (2017).
Benatti, P., Peluso, G., Nicolai, R. & Calvani, M. Polyunsaturated FA: Biochemical, nutritional, and epigenetic properties. J. Am. Coll. Nutr. 23, 281–302. https://doi.org/10.1080/07315724.2004.10719371 (2004).
Girard, J., Ferré, P., Pégorier, J. P. & Duée, P. H. Adaptations of glucose and fatty acid metabolism during perinatal period and suckling-weaning transition. Physiol. Rev. 72, 507–562. https://doi.org/10.1152/physrev.1992.72.2.507 (1992).
Odle, J., Lin, X., Van Kempen, T. A., Drackley, J. K. & Adams, S. H. Carnitine palmitoyl transferase modulation of hepatic fatty acid metabolism and radio-HPLC evidence for low ketogenesis in the neonatal pig. J. Nutr. 125, 2541–2549 (1995).
Oden, J. & Treen, W. R. Inborn errors of fasting adaptation. In B (ed. Nutrition in Pediatrics. W. A. Walker, J. B. Wakins, and C. Duggan,) 591–608 (C. Decker Inc., 2003).
Melendez, P. et al. Effect of fish oil and canola oil supplementation on immunological parameters, feed intake, and growth of Holstein calves. J. Dairy Sci. 105, 2509–2520. https://doi.org/10.3168/jds.2021-21134 (2022).
Makizadeh, H. et al. Corn-processing and crude protein content in calf starter: Effects on growth performance, ruminal fermentation, and blood metabolites. J. Dairy Sci. 103, 9037–9053. https://doi.org/10.3168/jds.2020-18578 (2020).
Puhakainen, I., Ahola, I. & Yki-Järvinen, H. Dietary supplementation with n-3 fatty acids increases gluconeogenesis from glycerol but not hepatic glucose production in patients with non-insulin-dependent diabetes mellitus. Am. J. Clin. Nutr. 61, 121–126. https://doi.org/10.1093/ajcn/61.1.121 (1995).
Lam, T. K. et al. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am. J. Physiol. Endocrinol. Metab. 284, E863-873. https://doi.org/10.1152/ajpendo.00033.2003 (2003).
Kazemi-Bonchenari, M. et al. Interactions between levels of heat-treated soybean meal and prilled fat on growth, rumen fermentation, and blood metabolites of Holstein calves. J. Anim. Sci. 94, 4267–4275. https://doi.org/10.2527/jas.2016-0514 (2016).
Kazemi-Bonchenari, M., Salem, A. Z. M. & Lopez, S. Influence of barley grain particle size and treatment with citric acid on digestibility, ruminal fermentation and microbial protein synthesis in Holstein calves. Animal. 11, 1295–1302. https://doi.org/10.1017/S1751731116002810 (2017).
Fokkink, W., Hill, T., Bateman, H., Aldrich, J. & Schlotterbeck, R. Selenium yeast for dairy calf feeds. Anim. Feed. Sci. Tech. 153, 228–235. https://doi.org/10.1016/j.anifeedsci.2009.06.015 (2009).
Calder, P. C., Yaqoob, P., Thies, F., Wallace, F. A. & Miles, E. A. Fatty acids and lymphocyte function. Br. J. Nutr. 87, 31–48. https://doi.org/10.1079/bjn2001455 (2002).
Kazemi-Bonchenari, M. et al. Effects of linseed oil and rumen-undegradable protein: Rumen-degradable protein ratio on performance of Holstein dairy calves. Br. J. Nutr. 123, 1247–1257. https://doi.org/10.1017/S0007114520000586 (2020).
Acknowledgements
The authors would like to thank Kimya Danesh Alvand Company for funding the research (grant number 2431) and for fatty acid analysis and provision of lipid preparations. In addition, the extensive cooperation with Moghan Agro-Industrial and Animal Breeding Complex, which allowed the experiment to be conducted with calves, is very valuable and commendable. The authors have not stated any conflicts of interest.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
The study was conducted by H.K.B., and B.M. The research was designed by H.K.B., B.M., M.K.B., and M.D.B.. M.H.G. acted as scientific advisors, performed data analysis and interpretation, and revised the manuscript. The final manuscript was read and approved by all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khalilvandi-Behroozyar, H., Mohtashami, B., Dehghan-Banadaky, M. et al. Effects of fat source in calf starter on growth performance, blood fatty acid profiles, and inflammatory markers during cold season. Sci Rep 13, 18627 (2023). https://doi.org/10.1038/s41598-023-45956-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45956-w
This article is cited by
-
Effects of low-quality forage and starter protein content in starter diet of young calves on growth performance, rumen fermentation, and urinary purine derivatives
Tropical Animal Health and Production (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.