Abstract
Primary mediastinal germ cell tumor (MGCT) is an uncommon tumor. Although it has histology similar to that of gonadal germ cell tumor (GCT), the prognosis for MGCT is generally worse than that for gonadal GCT. We performed visual assessment and quantitative analysis of [18F]fluorodeoxyglucose positron emission tomography/computed tomography ([18F]FDG PET/CT) for MGCTs. A total of 35 MGCT patients (age = 33.1 ± 16.8 years, F:M = 16:19) who underwent preoperative PET/CT were retrospectively reviewed. The pathologic diagnosis of MGCTs identified 24 mature teratomas, 4 seminomas, 5 yolk sac tumors, and 2 mixed germ cell tumors. Visual assessment was performed by categorizing the uptake intensity, distribution, and contour of primary MGCTs. Quantitative parameters including the maximum standardized uptake value (SUVmax), tumor-to-background ratio (TBR), metabolic tumor volume (MTV), total lesion glycolysis (TLG), and maximum diameter were compared between benign and malignant MGCTs. On visual assessment, the uptake intensity was the only significant parameter for differentiating between benign and malignant MGCTs (p = 0.040). In quantitative analysis, the SUVmax (p < 0.001), TBR (p < 0.001), MTV (p = 0.033), and TLG (p < 0.001) showed significantly higher values for malignant MGCTs compared with benign MGCTs. In receiver operating characteristic (ROC) curve analysis of these quantitative parameters, the SUVmax had the highest area under the curve (AUC) (AUC = 0.947, p < 0.001). Furthermore, the SUVmax could differentiate between seminomas and nonseminomatous germ cell tumors (p = 0.042) and reflect serum alpha fetoprotein (AFP) levels (p = 0.012). The visual uptake intensity and SUVmax on [18F]FDG PET/CT showed discriminative ability for benign and malignant MGCTs. Moreover, the SUVmax may associate with AFP levels.
Similar content being viewed by others
Introduction
Primary mediastinal germ cell tumors (MGCTs) are uncommon tumors that account for 2–4% of all germ cell tumors (GCTs), which may be found predominantly in young males with markedly elevated tumor markers such as alpha fetoprotein (AFP) or human chorionic gonadotropin (HCG)1,2. Due to histological resemblance, primary MGCTs can be classified in the same way as GCTs. Mature teratoma is generally considered as benign MGCT, and malignant MGCTs can be broadly subdivided into seminomas and nonseminomatous germ cell tumors (NSGCTs)3,4. Different NSGCT entities include immature teratoma, seminoma, yolk sac tumor, choriocarcinoma, and embryonal carcinoma. When more than one type of histology is present, the tumor may be considered as mixed MGCT (malignant MGCT entity). Although MGCTs have similar histology similar to that of gonadal GCTs, the prognosis for MGCTs is generally worse than that for gonadal GCTs; however, studies of MGCTs have shown that patients with seminomas have a more favorable prognosis than that of patients with NSGCTs5.
There have been no prospective studies to define the diagnostic approach, prognostic stratification, and treatment strategies for MGCTs, and only a few retrospective studies and case series exist6. Therefore, the treatment and management of this disease remain challenging and require a multidisciplinary approach. The tumor location, extension, serum tumor markers, and histopathological type are considered critical information for the diagnosis of and treatment planning for GCTs7.
Recently, the role of [18F]fluorodeoxyglucose positron emission tomography/computed tomography ([18F]FDG PET/CT) in the diagnosis and prognostic stratification of mediastinal tumors has been investigated8,9. However, there are limited [18F]FDG PET/CT-related studies on primary MGCTs, and those that have been published mainly consist of case reports and small series. A few studies reported that [18F]FDG PET/CT had additional value for detecting distant metastasis or recurrence after chemotherapy10,11. Other studies reported that [18F]FDG PET/CT parameters showed positive associations with tumor markers such as AFP and HCG12,13. In another preliminary study, the tumor-to-mediastinal ratio of [18F]FDG PET/CT was found to be significantly correlated with the expression of Glut1, HIF-1, EGFR, p-Akt, and p-S6K in primary non-thymic neoplasms14. However, no structured study has conducted about the visual assessment or quantitative analysis of [18F]FDG PET/CT parameters for MCGTs.
We examined the usefulness of visual assessment and quantitative analysis of [18F]FDG PET/CT in primary MGCT differentiation. In addition, we investigated the association between serum tumor markers and quantitative PET/CT parameters.
Results
Patient characteristics
A total of 35 consecutive patients (16 females and 19 males) were included in the analysis. The median patient age at the time of PET/CT image acquisition was 27 years. The median time interval between biopsy or surgery and PET/CT was 13 days (range, 0–134 days). The patients were finally pathologically diagnosed with MGCTs through excisional biopsy (n = 24) or resective surgery (n = 11). A total of 24 patients had benign mature teratomas, and 11 patients had malignant lesions, including seminomas (n = 4), yolk cell tumors (n = 5), and mixed GCTs (n = 2) (Table 1).
In comparison with patients with benign MGCTs, patients with malignant MGCTs were significantly younger (38.2 ± 18.0 years vs. 21.9 ± 4.0 years, p < 0.001). All 11 patients in the malignant group were male. The time interval between surgery and PET/CT was not significantly different between benign and malignant MGCTs (p = 0.221). The tumor markers AFP and HCG were examined in 26 of the 35 patients, and AFP was found to be significantly higher in patients with malignant MGCTs than in patients with benign MGCTs (p = 0.004), whereas HCG showed no significant difference (p = 0.604) (Table 2).
Visual assessment
All 10 grade 1 or 2 cases involved benign MGCTs, and all malignant MGCTs showed grade 3 uptake. The uptake intensity could differentiate between benign and malignant MGCTs (p = 0.040). The distribution and contour were not significant in differentiating between benign and malignant MGCTs (p = 0.146 and 0.331, respectively) (Table 3).
Quantitative analysis
The median values of the PET/CT parameters of all patients were SUVmax 3.3, TBR 3.2, MTV 12.6 cm3, and TLG 18.8. The PET/CT parameters SUVmax, TBR, MTV, and TLG were significantly higher in the malignant MGCT group than in the benign MGCT group (p < 0.001, p < 0.001, p = 0.033, and p < 0.001, respectively), whereas the maximum diameter did not show a significant difference (p = 0.115) between the two groups (Table 4 and Fig. 1).
In ROC curve analysis of the evaluated parameters, the SUVmax had the highest AUC (AUC = 0.947, cut-off > 4.5) and demonstrated significantly better diagnostic performance than that of the MTV and maximum diameter in discriminating between benign and malignant MGCTs. A specific cut-off value of 4.5 for the SUVmax showed sensitivity of 81.8% and specificity of 100.0% in differentiating between benign and malignant MGCTs. The TBR (AUC = 0.917, cut-off > 4.9) and TLG (AUC = 0.920, cut-off > 24.6) also showed significant discriminative performance in differentiating between benign and malignant MGCTs; however, the AUC was not significantly different compared with that of the SUVmax (Table 5 and Fig. 2).
Representative 18F-FDG PET/CT cases of mediastinal germ cell tumors (MGCTs) with the pathologic subtype. (a) A 21-year-old man with benign MGCT (mature teratoma) showing grade 1 uptake, homogenous distribution, and round contour (SUVmax 1.9, arrow). (b) A 31-year-old man with seminoma showing grade 3 uptake, heterogenous distribution, and lobulated contour (SUVmax 15.0, arrow). (c) A 22-year-old man with yolk cell tumor showing grade 3 uptake, heterogenous distribution, and infiltrative contour (SUVmax 18.7, arrow) with anterior pleural invasion, pleural effusion, and bone metastasis in the sternum (arrowheads).
Comparison of quantitative parameters between seminomas and NSGCTs
Malignant lesions were subdivided into seminomas and NSGCTs for subgroup analysis. Among the evaluated parameters, the SUVmax, TBR, and maximum diameter showed significant differences between the two subgroups (p = 0.042, p = 0.042, and p = 0.012, respectively) (Table 4).
Association between quantitative parameters and tumor markers
Patients were divided into two groups (high and low) according to the threshold of the median value of each tumor marker (AFP = 2 and HCG = 1). The SUVmax, TBR, and maximum diameter were significantly higher in the high AFP group (p = 0.012, p = 0.034, and p = 0.044, respectively) (Table 6); however, no PET/CT parameters showed significant differences between the high and low HCG groups (Supplementary Table 1).
Discussion
In this study, distinctive features of MGCTs were identified using the visual uptake intensity on [18F]FDG PET/CT. All malignant MGCTs showed grade 3 uptake. In addition, the information provided by the quantitative [18F]FDG PET/CT parameters showed potential for discriminating benign MGCTs from malignant MGCTs. All quantitative PET parameters showed significantly higher values for malignant MGCTs compared with MGCTs. Among the parameters evaluated, the SUVmax had the highest AUC value and exhibited superior diagnostic performance than that of the MTV and maximum diameter. Furthermore, the SUVmax was significantly higher for NSGCTs and the high AFP subgroup.
MGCT is a disease with heterogeneous entities, accounting for approximately 14–24% of mediastinal tumors15,16 with high prevalence in young adults and children17. The role of [18F]FDG PET/CT in the initial diagnosis of MGCTs has not been established, which needs to be further investigated6. In terms of visual analysis, a review indicated that benign MGCTs could show mild-to-moderate uptake and that malignant MGCTs could demonstrate higher uptake than that of benign MGCTs, which are consistent with our study results18. Based on visual observations, we found that the increased uptake of [18F]FDG PET/CT higher than that of the liver could be a potential sign of malignant MGCTs. In contrast, the distribution and contour showed no significant difference19, indicating that the uptake intensity could be a better visual assessment parameter. In terms of quantitative analysis, the SUVmax had a higher AUC value than that of volumetric parameters such as the MTV or TLG in the differentiation of malignant and benign MGCTs. Therefore, the oncologic behavior of MGCTs might be largely dependent on which malignant component of the GCT histology is included rather than the whole volume of tumor cells, such as in the case of thymic epithelial tumors20.
Among malignant MGCTs, seminomas account for two-thirds of tumors, and NSGCTs account for the remaining one-third21. The prognosis of NSGCTs is worse than that of seminomas; thus, differentiation between these two entities is important5. As [18F]FDG PET/CT reflects the glycolysis pathway, high SUVmax is usually correlated with aggressiveness and proliferative activity22. Our results demonstrated the possibility of discrimination between seminomas and NSGCTs using the SUVmax.
A recent study reported that for patients with elevated (at least two) tumor markers after surgery, [18F]FDG PET/CT had a higher positive predictive value in the primary staging of testicular GCTs12. The authors also reported that the MTV and TLG showed significant positive correlations with HCG, whereas AFP showed a significant correlation only for patients with NSGCTs. Similarly, our study showed significance between tumor marker levels and quantitative parameters; however, a positive association was only observed with AFP levels. Differences between the studies could be explained by the heterogeneity of patients included in each study23. Specifically, differences in the proportions of subtypes in each study could have led to different results considering that seminomas rarely produce AFP but may have a variable amount of HCG; in contrast, high AFP levels in yolk cell tumors and high HCG levels in choriocarcinomas are common and strongly correlated with the whole tumor volume.
This study has several limitations. First, as this was a single-center retrospective study, the possibility of referral bias cannot be excluded. Second, some of the patients did not have tumor marker results and were excluded from subgroup analysis. Finally, as our sample size was small, other tumor markers including lactate dehydrogenase, which is usually elevated in malignant MGCTs, could not be evaluated. A prospective, multicenter study with a larger number of patients is needed in the future.
In conclusion, the visual uptake intensity of MGCTs on [18F]FDG PET/CT was useful for discriminating between benign and malignant MGCTs. Additionally, the SUVmax on [18F]FDG PET/CT could provide information for differentiating between benign and malignant MGCTs. Specifically, the SUVmax could differentiate seminomas and NSGCTs among malignant MGCTs and may be associated with AFP levels.
Methods
Study design and subjects
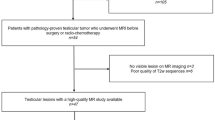
We retrospectively reviewed the medical records of 117 patients who underwent biopsy or surgery for MGCTs at our institution between January 2010 and December 2021. Among these patients, 40 patients who underwent [18F]FDG PET/CT within four months prior to biopsy or surgery were identified. Five patients who underwent PET/CT at an other hospital and who did not have all the information needed for the quantification of [18F]FDG PET/CT data (i.e., radiotracer injection dose, injection-to-scan time) were excluded from this study (Fig. 3). This study was approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB no. S2021-2042-0001), and all methods were performed in accordance with the relevant guidelines and regulations. The need for informed consent was waived by the IRB of Asan Medical Center because of the retrospective nature of the study.
[18F]FDG PET/CT image acquisition
All of the studied patients fasted for at least 6 h before [18F]FDG PET/CT image acquisition, and their venous blood glucose levels were maintained below 150 mg/dl. Patients were positioned in the scanners with their arms above their heads. [18F]FDG PET/CT was performed using one of the following PET/CT scanners: Biograph TruePoint 40 (Siemens Healthineers, Erlangen, Germany), Discovery 690, Discovery 690 Elite, or Discovery 710 (GE Healthcare, Milwaukee, WI, USA). The patients were intravenously administered 5.2 MBq/kg [18F]FDG, and PET emission images were obtained 1 h after the injection, with 5–6 bed positions covering the skull base to the upper thigh. The PET parameters included 2.5 min/bed and 168 × 168 matrix size (Biograph TruePoint 40) or 2 min/bed and 192 × 192 matrix size (Discovery series) in the 3D acquisition mode. The CT acquisition parameters were 120 kVp, 10 mA, and a 5 mm slice thickness (Biograph TruePoint 40) or 140 kVp, Auto mA, and a 3.75 mm slice thickness (Discovery series). PET images were reconstructed using a 3D ordered-subset expectation maximization (OSEM) algorithm with attenuation correction based on the CT data.
[18F]FDG PET/CT image analysis
Both visual assessment and quantitative analyses were performed by two researchers (K. L. and Y-i. K.) who were blinded to the clinical information. The PET/CT images were visually assessed, and each case was categorized according to the [18F]FDG uptake intensity, distribution, and contour of the primary MGCT. For image interpretation, the uptake intensity grade was defined as follows: grade 1, lower or equal to mediastinal uptake; grade 2, greater than mediastinal uptake but lower or equal to liver uptake; grade 3, greater than liver uptake. The distribution was defined as homogenous (uniform uptake and shape) or heterogeneous (uneven uptake and shape). The contour was defined as round (similar width and length with smooth margin), lobulated (smooth margin), or infiltrative (irregular margin). All categories were evaluated using PET, CT, and PET/CT fusion images.
For the quantitative analysis of PET/CT images, which included data from different scanners, the SUVs were equalized across the scanners. Using an American College of Radiology-approved Esser phantom (Data Spectrum, Hillsborough, NC, USA) filled with [18F]FDG water solution to set the SUVs of the hot cylinders to 2.5 and the background to 1.0, the SUVs of different hot cylinders with varying diameters were measured, and recovery coefficient (i.e., SUV ratios relative to the ideal SUV of 2.5) plots were generated. Recovery coefficients allowed the estimation of the optimal smoothing kernel size for each matched recovery coefficient generated. After harmonization, the volume-of-interest (VOI) of the mediastinal mass was drawn on PET/CT fusion images, which allowed the VOI to be drawn within the mass shown on the CT images. All PET/CT parameters obtained from Biograph TruePoint 40 data were calculated by drawing one VOI using the Mirada DBx workstation (version 1.2.0.59; Mirada Medical Ltd., Oxford, UK). Harmonized SUV values from the Discovery series data were measured using the in-house software ‘AMC NM Toolkit for Image Quantification of Excellence (ANTIQUE)’ with manually traced VOIs24,25,26.
The maximum standardized uptake value (SUVmax) was the highest pixel uptake in the mediastinal mass, and the tumor-to-background ratio (TBR) was calculated as the SUVmax of the mediastinal mass divided by the mean SUV (SUVmean) of the aorta within 2 cm3. The volumetric parameter metabolic tumor volume (MTV) was segmented by applying a threshold of more than 50% of the relative value of the SUVmax within the VOI, and the total lesion glycolysis (TLG) was calculated as the SUVmean multiplied by the MTV. The maximum diameter of the tumor was measured on a combined transaxial CT image.
Statistical analysis
Continuous parameters were expressed as mean ± standard deviation with range for normal distribution and included median when the values were non-normal distribution. Categorical parameters were analyzed by Pearson’s chi-square test or Fischer’s exact test to differentiate between benign and malignant MGCTs. Continuous parameters were analyzed by independent-samples t-test or Mann–Whitney U test. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic performance for benign and malignant MGCTs. DeLong’s method was used to compare the area under the curve (AUC) values and their 95% confidence intervals (CIs). Optimal cut-off values were defined as the exploratory cut-off values with the highest accuracy based on the Youden Index. All statistical analyses were performed using SPSS software version 17 (SPSS, Chicago, IL) and MedCalc version 19.2.3 (MedCalc Software Ltd., Ostend, Belgium).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bokemeyer, C. et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: Results from an international analysis. J. Clin. Oncol. 20, 1864–1873 (2002).
Hainsworth, J. D. & Greco, F. A. Extragonadal germ cell tumors and unrecognized germ cell tumors. Semin. Oncol. 19, 119–127 (1992).
Azizad, S. et al. Solid tumors of the mediastinum in adults. Semin. Ultrasound. CT MR. 37, 196–211 (2016).
Yang, C. et al. Primary germ cell tumors of the mediastinum: 10 years of experience in a tertiary teaching hospital. Kaohsiung J. Med. Sci. 21, 395–400 (2005).
Koizumi, T. et al. Primary mediastinal germ cell tumors: A retrospective analysis of >30 years of experience in a single institution. Thorac. Cancer. 12, 807–813 (2021).
Kumar, N. et al. Primary mediastinal germ cell tumors: Survival outcomes and prognostic factors: 10 years experience from a tertiary care institute. Rare Tumors. 12, 2036361320972220. https://doi.org/10.1177/2036361320972220 (2020).
Mishra, S. et al. “Primary malignant mediastinal germ cell tumors: A single institutional experience. South Asian J. Cancer. 9, 27–29 (2020).
Han, S. et al. Fully automatic quantitative measurement of 18F-FDG PET/CT in thymic epithelial tumors using a convolutional neural network. Clin. Nucl. Med. 47, 590–598 (2022).
Han, S. et al. Diagnostic and prognostic values of 2-[18F]FDG PET/CT in resectable thymic epithelial tumour. Eur. Radiol. 32, 1173–1183 (2022).
De Giorgi, U. et al. FDG-PET in the management of germ cell tumor. Ann. Oncol. 16(Suppl 4), 90–94 (2005).
de Wit, M. et al. [18F]-FDG-PET in clinical stage I/II non-seminomatous germ cell tumours: results of the German multicentre trial. Ann. Oncol. 19, 1619–1623 (2008).
Aydos, U. et al. 18F-FDG PET/CT for primary staging of patients with testicular germ cell tumors: the predictors of 18F-FDG PET positivity and prognostic value of PET derived metabolic parameters. Nucl. Med. Commun. 41, 1199–1209 (2020).
Alongi, P. et al. Diagnostic and prognostic value of 18F-FDG PET/CT in recurrent germinal tumor carcinoma. Eur. J. Nucl. Med. Mol. Imaging. 45, 85–94 (2018).
Kaira, K. et al. 18F-FDG uptake on PET in primary mediastinal non-thymic neoplasm: A clinicopathological study. Eur. J. Radiol. 81, 2423–2429 (2012).
Wright, C. D. Mediastinal tumors and cysts in the pediatric population. Thorac. Surg. Clin. 19, 47–61 (2009).
Pearson, J. K. & Tan, G. M. Pediatric anterior mediastinal mass: A review article. Semin. Cardiothorac. Vasc. Anesth. 19, 248–254 (2015).
Jaggers, J. & Balsara, K. Mediastinal masses in children. Semin. Thorac. Cardiovasc. Surg. 16, 201–208 (2004).
Sharma, P. et al. Evaluation of thymic tumors with 18F-FDG PET-CT: A pictorial review. Acta Radiol. 54, 14–21 (2013).
Carter, B. W. et al. Approaching the patient with an anterior mediastinal mass: A guide for clinicians. J. Thorac. Oncol. 9(9 Suppl 2), S102-109 (2014).
Lee, H. S. et al. Differentiating the grades of thymic epithelial tumor malignancy using textural features of intratumoral heterogeneity via (18)F-FDG PET/CT. Ann. Nucl. Med. 30, 309–319 (2016).
Moran, C. A. & Suster, S. Primary germ cell tumors of the mediastinum: I. Analysis of 322 cases with special emphasis on teratomatous lesions and a proposal for histopathologic classification and clinical staging. Cancer. 80, 681–690 (1997).
Treglia, G. et al. Is (18)F-FDG PET useful in predicting the WHO grade of malignancy in thymic epithelial tumors? A meta-analysis. Lung Cancer. 86, 5–13 (2014).
Gilligan, T. D. et al. American society of clinical oncology clinical practice guideline on uses of serum tumor markers in adult males with germ cell tumors. J. Clin. Oncol. 28, 3388–3404 (2010).
Oh, J. S. et al. Intratumor textural jeterogeneity on pretreatment (18)F-FDG PET Images predicts response and survival after chemoradiotherapy for hypopharyngeal cancer”. Ann. Surg. Oncol. 22, 2746–2754 (2015).
Ha, S. C. et al. Pretreatment tumor SUVmax predicts disease-specific and overall survival in patients with head and neck soft tissue sarcoma. Eur. J. Nucl. Med. Mol. Imaging. 44, 33–40 (2017).
Kim, J. W. et al. Prognostic significance of standardized uptake value and metabolic tumour volume on 18F-FDG PET/CT in oropharyngeal squamous cell carcinoma. Eur. J. Nucl. Med. Mol Imaging. 42, 1353–1361 (2015).
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HR18C0016).
Author information
Authors and Affiliations
Contributions
K.L. performed the primary PET/CT image analysis and prepared the manuscript. Y.-i.K. conceptualized the study design and prepared the manuscript. J.S.O. and S.Y.S. performed PET/CT image normalization. J.K.Y., G.D.L., S.C., H.R.K., Y.-H.K., D.K.K., and S.-I.P. participated in patient recruitment and gathered patients’ clinical data. J.-S.R. provided critical analysis and revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, K., Kim, Yi., Oh, J.S. et al. [18F]fluorodeoxyglucose positron emission tomography/computed tomography characteristics of primary mediastinal germ cell tumors. Sci Rep 13, 17619 (2023). https://doi.org/10.1038/s41598-023-44913-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44913-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.